Synthesis of Copper Nanoparticles Using Glass Microfluidic Device †
Abstract
:1. Introduction
2. Materials and Methods
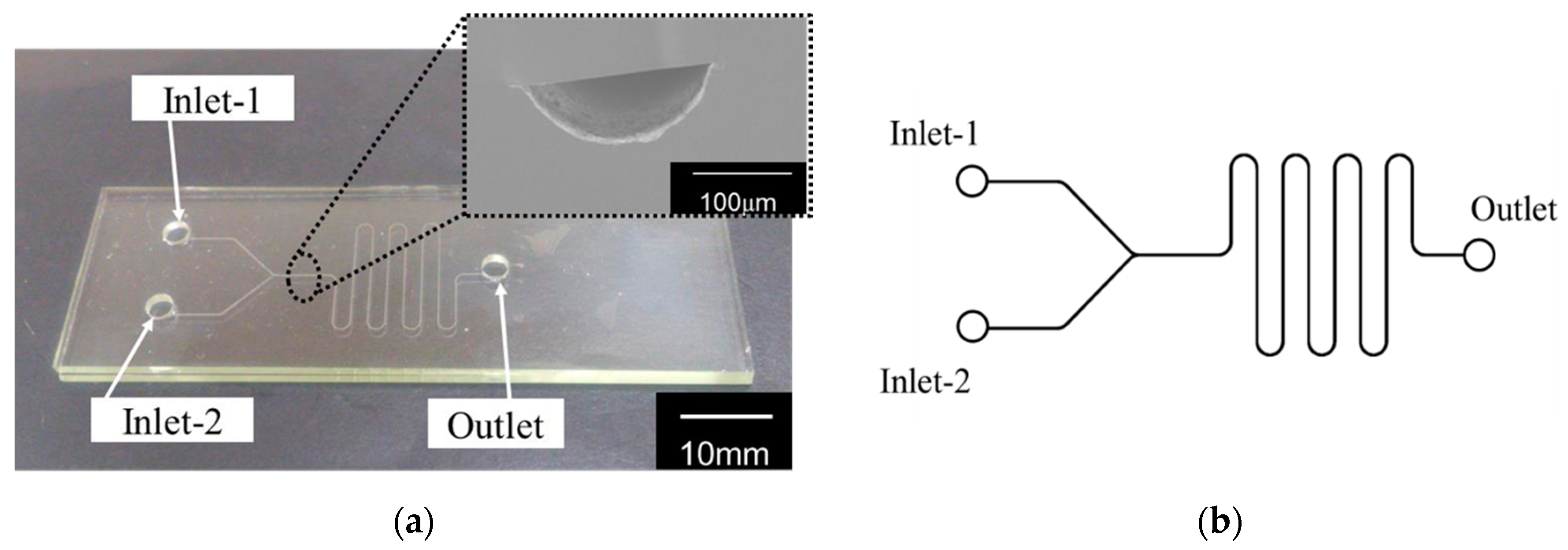
2.1. Glass Device
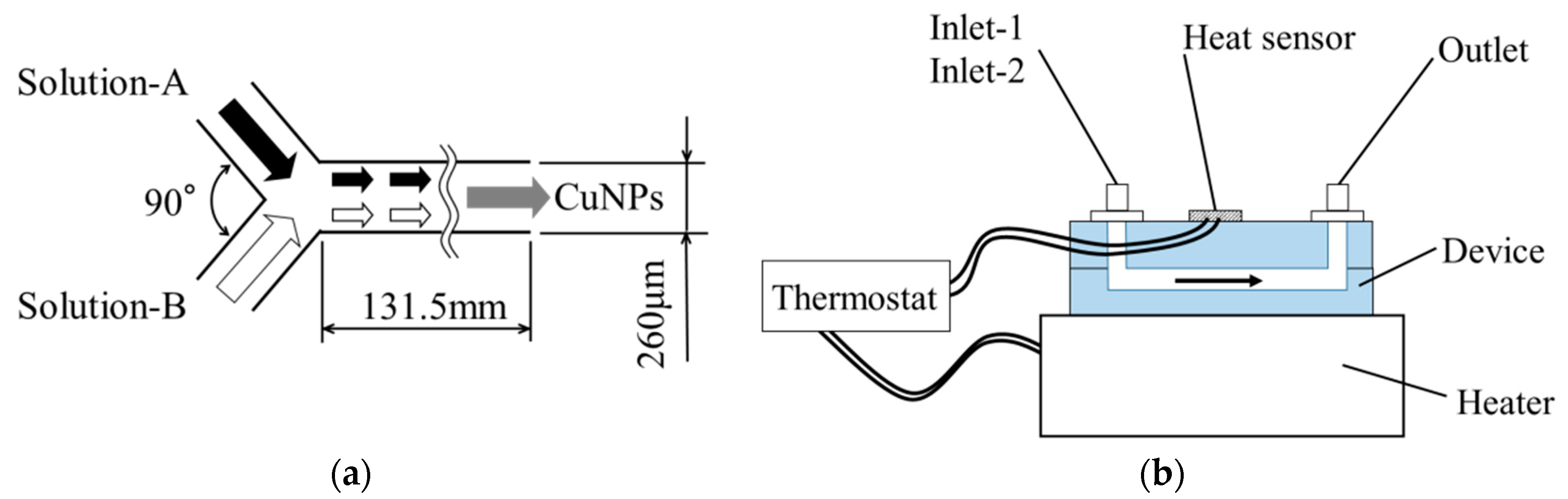
2.2. Synthesis
2.3. Characterization
3. Results and Discussion
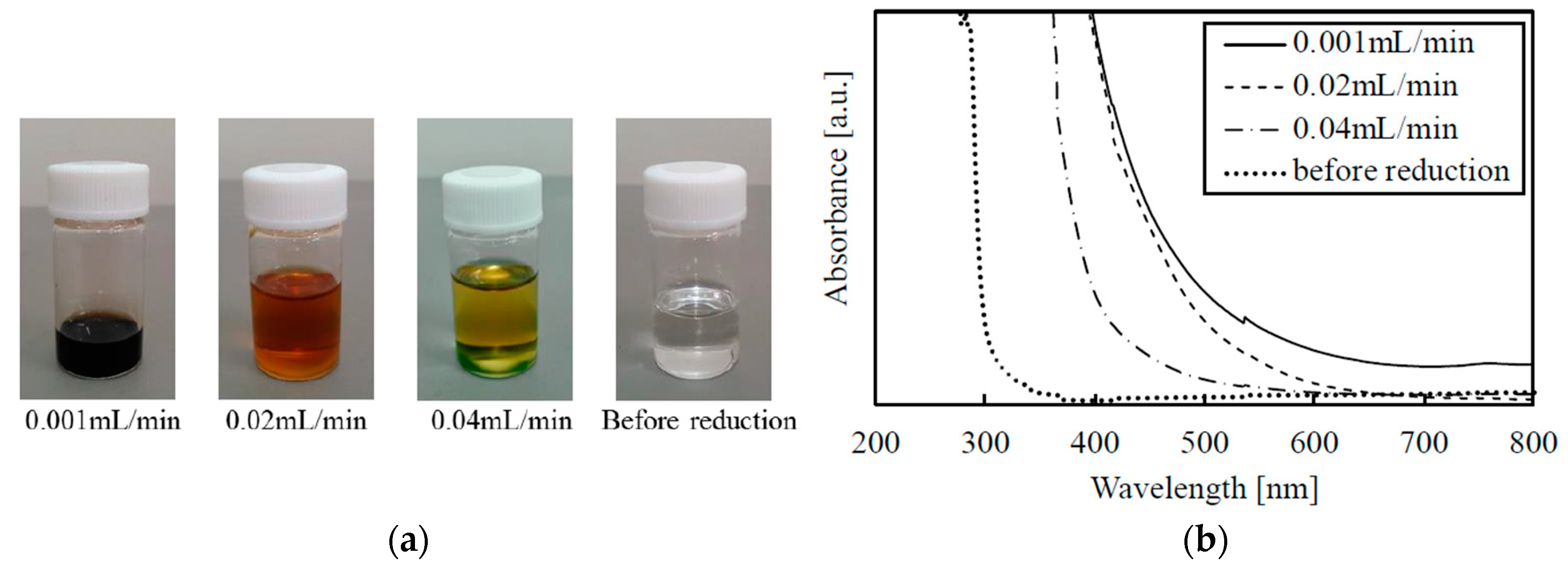
3.1. Absorption Spectrum
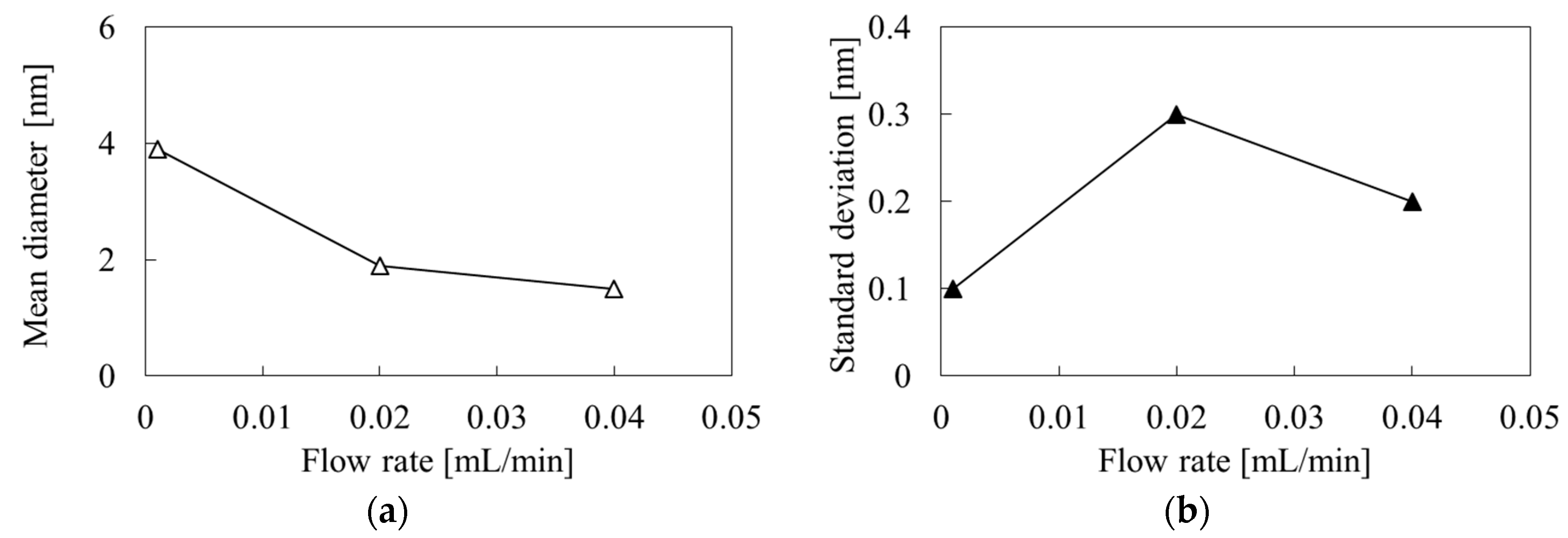
3.2. Dynamic Light Scattering Measurements
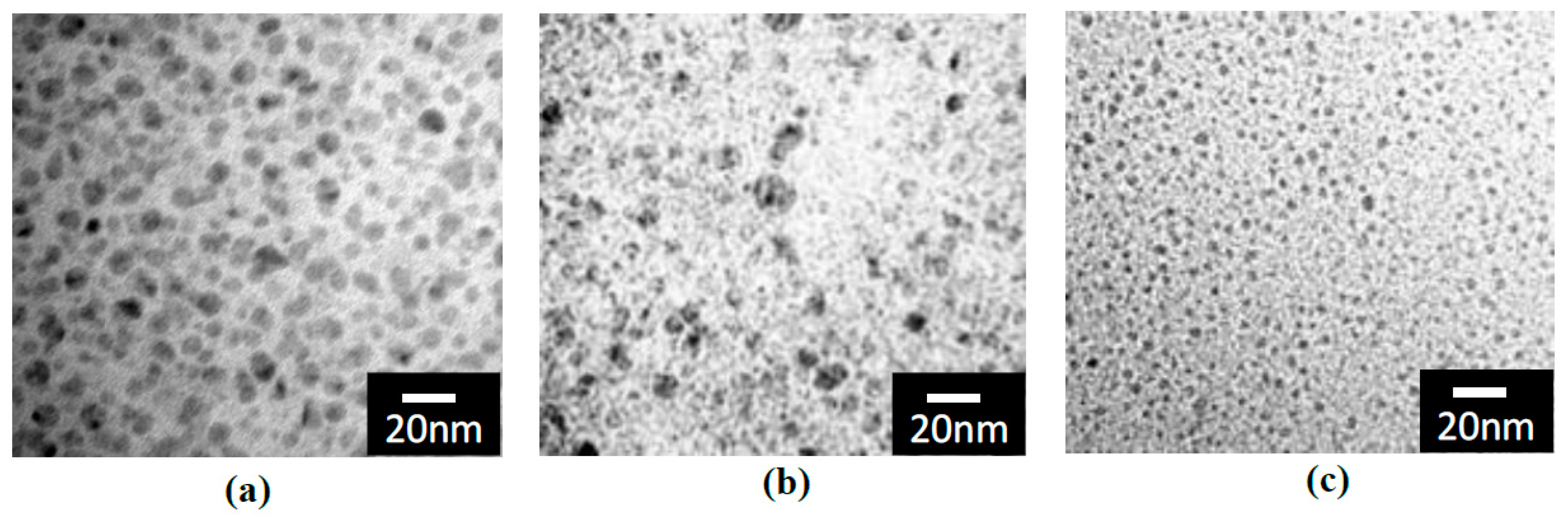
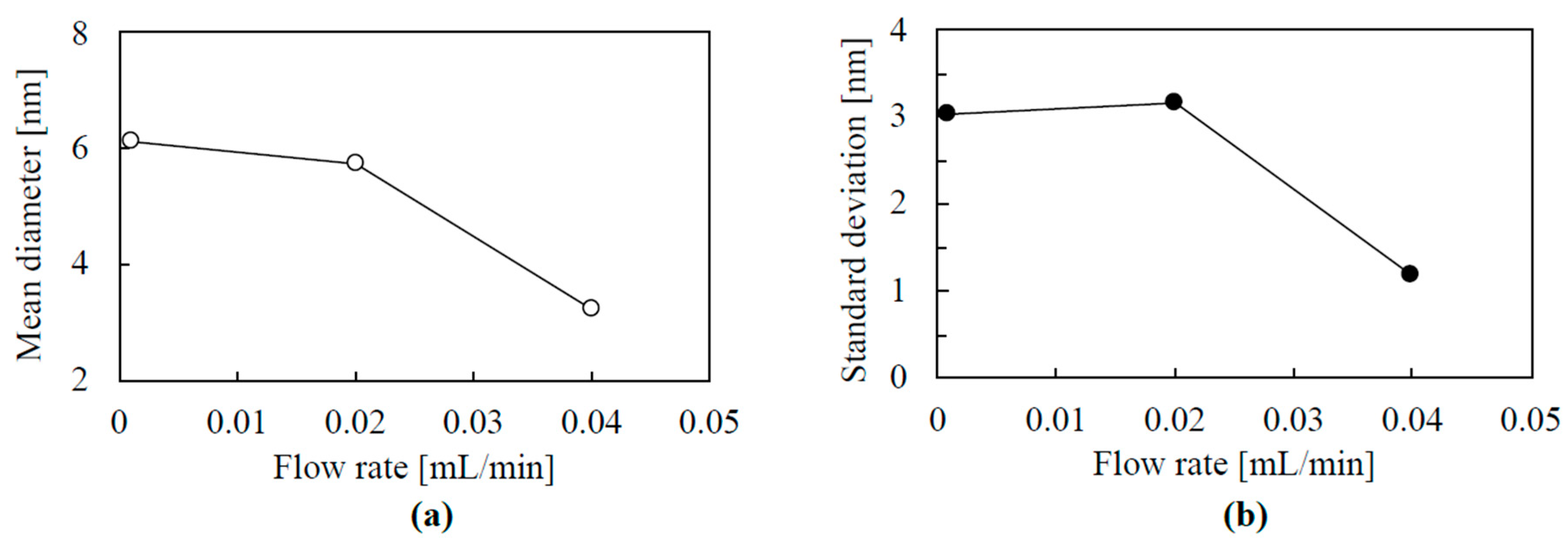
3.3. TEM Images
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Fuller, S.B.; Wilhelm, E.J.; Jacobson, J.M. Ink-jet Printed Nanoparticle Microelectromechanical Systems. J. Microelectromech. Syst. 2002, 11, 54–60. [Google Scholar] [CrossRef]
- Li, M.; Xiang, K.; Luo, G.; Gong, D.; Shen, Q. Preparation of Monodispersed Copper Nanoparticles by an Environmentally Friendly Chemical Reduction. Chin. J. Chem. 2013, 31, 1285–1289. [Google Scholar] [CrossRef]
- Wagner, J.; Kirner, T.; Albert, J.; Köhler, J.M. Generation of Metal Nanoparticles in a Microchannel Reactor, Chem. Eng. J. 2004, 101, 251–260. [Google Scholar]
- Yagyu, H.; Yu. Tanabe, Y.; Takano, S.; Hamamoto, M. Continuous Flow Synthesis of Monodisperse Gold Nanoparticles by Liquid-phase Reduction Method on Glass Microfluidic Device. Micro Nanno Lett. 2017, 12, 536–539. [Google Scholar] [CrossRef]
- Yagyu, H.; Sugano, K.; Hayashi, S.; Tabata, O. Micropowder Blasting using Nanoparticles Dispersed Polymer Mask for Rapid Prototyping of Glass Chip. J. Micromech. Microeng. 2005, 15, 1236–1241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Shinozaki, Y.; Yagyu, H. Synthesis of Copper Nanoparticles Using Glass Microfluidic Device. Proceedings 2018, 2, 1110. https://doi.org/10.3390/proceedings2131110
Liang Y, Shinozaki Y, Yagyu H. Synthesis of Copper Nanoparticles Using Glass Microfluidic Device. Proceedings. 2018; 2(13):1110. https://doi.org/10.3390/proceedings2131110
Chicago/Turabian StyleLiang, Yiyang, Yoko Shinozaki, and Hiromasa Yagyu. 2018. "Synthesis of Copper Nanoparticles Using Glass Microfluidic Device" Proceedings 2, no. 13: 1110. https://doi.org/10.3390/proceedings2131110
APA StyleLiang, Y., Shinozaki, Y., & Yagyu, H. (2018). Synthesis of Copper Nanoparticles Using Glass Microfluidic Device. Proceedings, 2(13), 1110. https://doi.org/10.3390/proceedings2131110




