Optical Ammonia Sensor for Continuous Bioprocess Monitoring †
Abstract
:1. Introduction
2. Materials and Methods
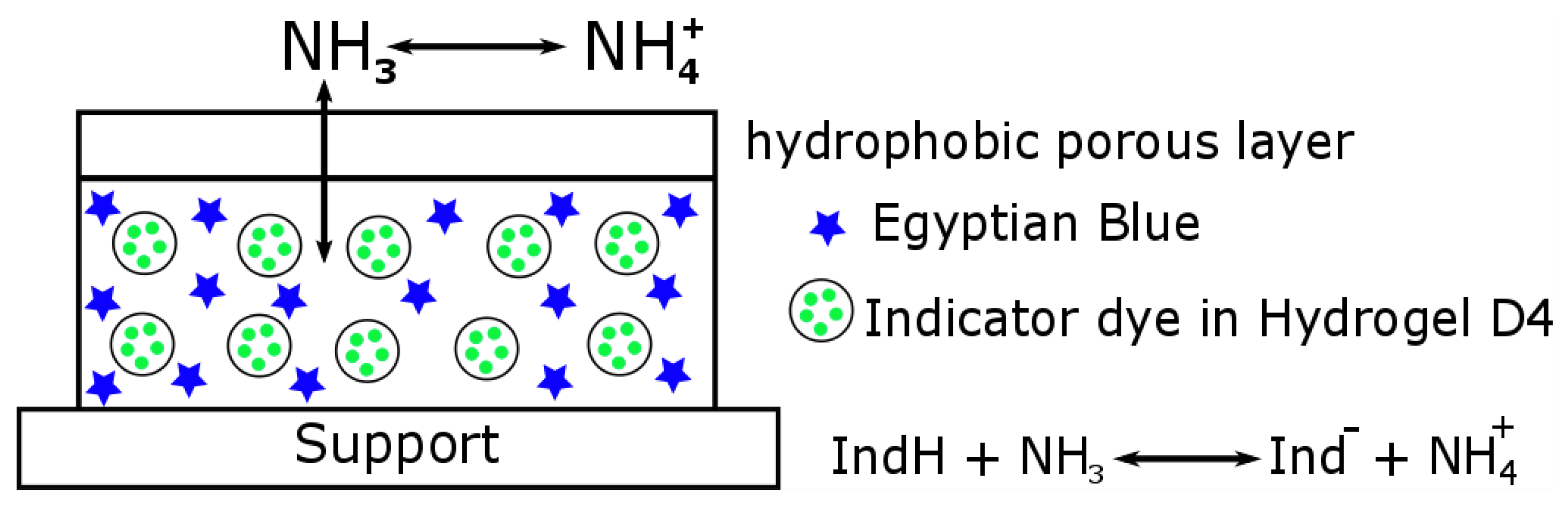
2.1. Sensor Preparation
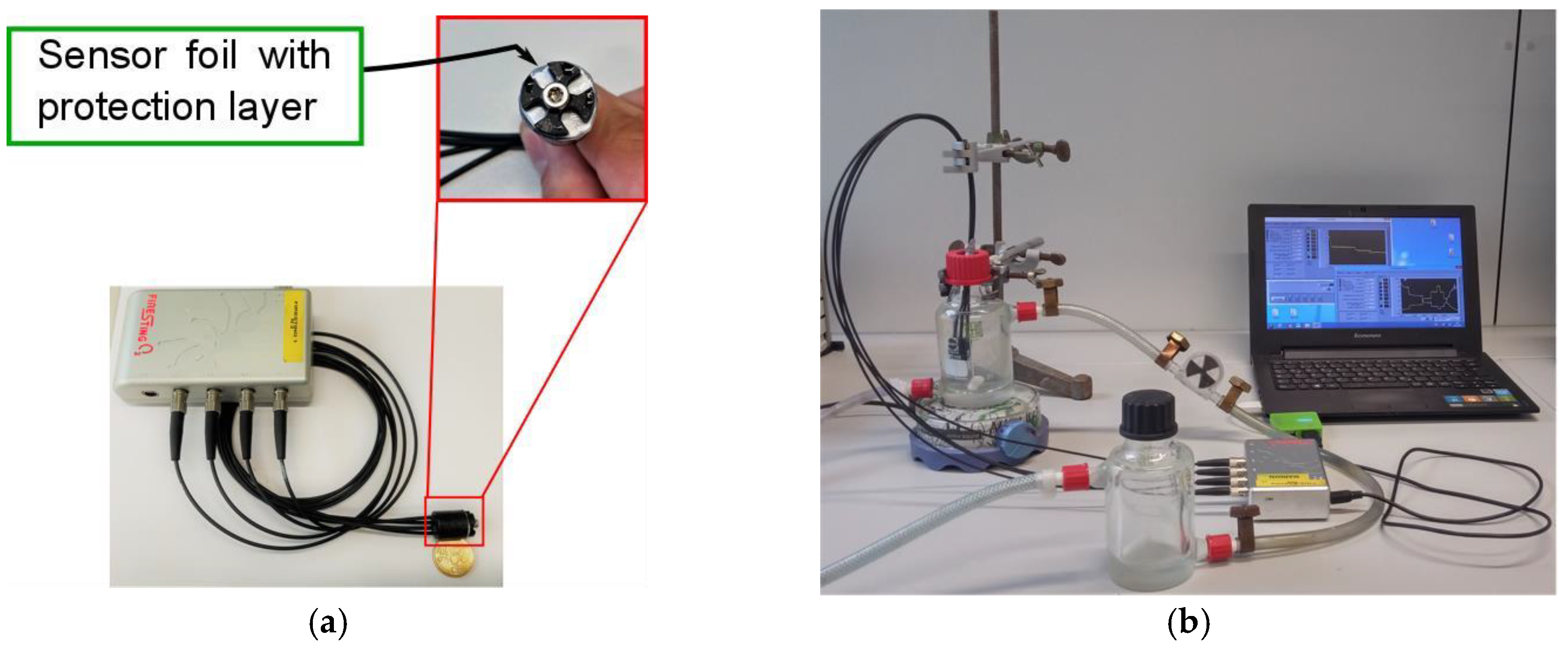
2.2. Measurement Set-Up
3. Results
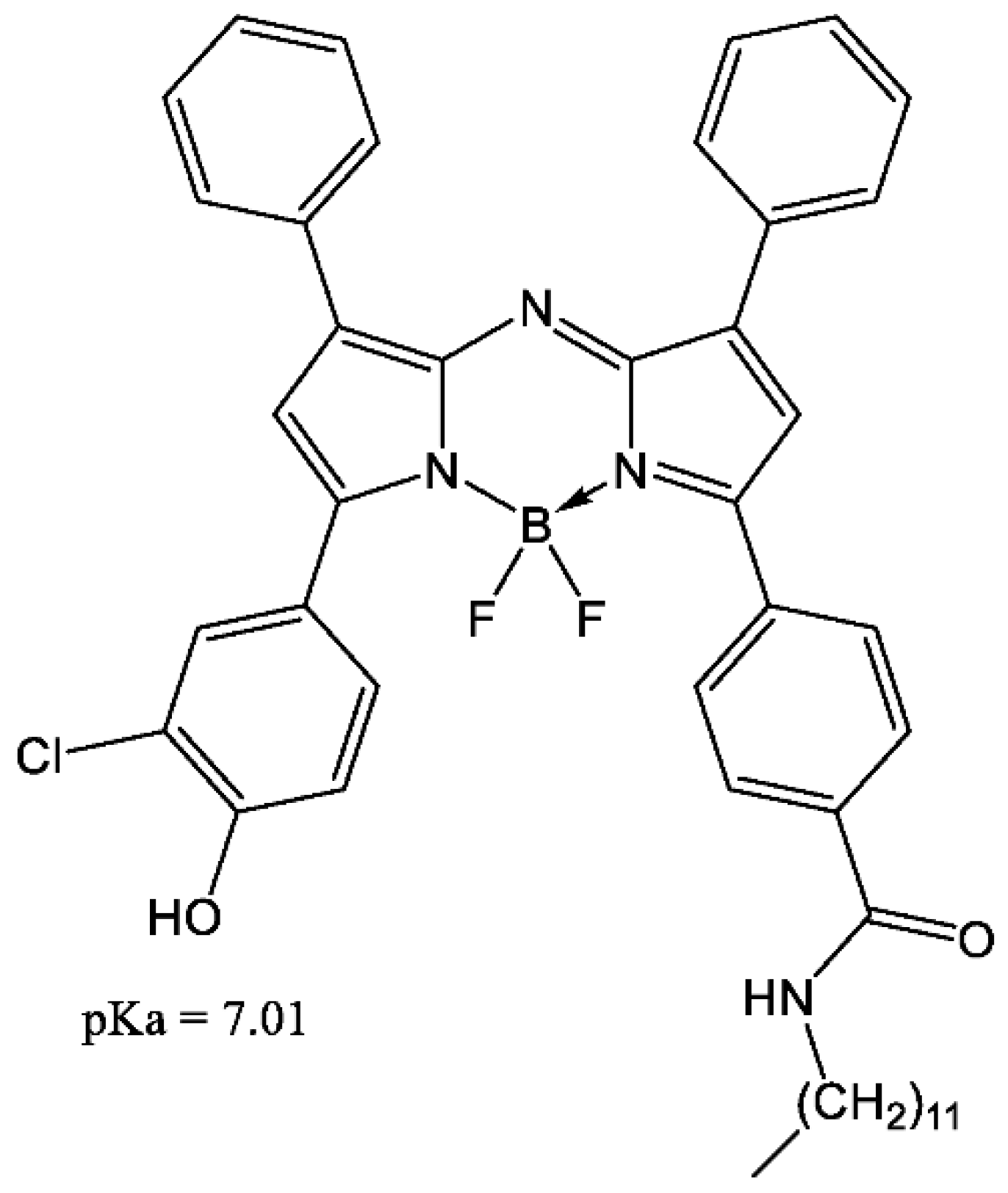
3.1. Choice of the Dye
3.2. Sensor Calibation and Response Time
4. Discussion and Conclusions
References
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Irani, N.; Wirth, M.; van den Heuvel, J.; Wagner, R. Improvement of the primary metabolism of cell cultures by introducing a new cytoplasmic pyruvate carboxylase reaction. Biotechnol. Bioeng. 1999, 66, 238–246. [Google Scholar] [CrossRef]
- Reitzer, L.J.; Wice, B.M.; Kennell, D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 1979, 254, 2669–2676. [Google Scholar] [CrossRef]
- Newland, M.; Greenfield, P.F.; Reid, S. Hybridoma growth limitations: The roles of energy metabolism and ammonia production. Cytotechnology 1990, 3, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Strobl, M.; Rappitsch, T.; Borisov, S.M.; Mayr, T.; Klimant, I. NIR-emitting aza-BODIPY dyes—New building blocks for broad-range optical pH sensors. Analyst 2015, 140, 7150–7153. [Google Scholar] [CrossRef] [PubMed]
- Strobl, M.; Walcher, A.; Mayr, T.; Klimant, I.; Borisov, S.M. Trace Ammonia Sensors Based on Fluorescent Near-Infrared-Emitting aza-BODIPY Dyes. Anal. Chem. 2017, 89, 2859–2865. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maierhofer, M.; Borisov, S.M.; Mayr, T. Optical Ammonia Sensor for Continuous Bioprocess Monitoring. Proceedings 2018, 2, 1041. https://doi.org/10.3390/proceedings2131041
Maierhofer M, Borisov SM, Mayr T. Optical Ammonia Sensor for Continuous Bioprocess Monitoring. Proceedings. 2018; 2(13):1041. https://doi.org/10.3390/proceedings2131041
Chicago/Turabian StyleMaierhofer, Maximilian, Sergey M. Borisov, and Torsten Mayr. 2018. "Optical Ammonia Sensor for Continuous Bioprocess Monitoring" Proceedings 2, no. 13: 1041. https://doi.org/10.3390/proceedings2131041
APA StyleMaierhofer, M., Borisov, S. M., & Mayr, T. (2018). Optical Ammonia Sensor for Continuous Bioprocess Monitoring. Proceedings, 2(13), 1041. https://doi.org/10.3390/proceedings2131041




