Design of a Photonic Crystal Defect Waveguide Biosensor Operating in Aqueous Solutions at 1.34 µm †
Abstract
1. Introduction
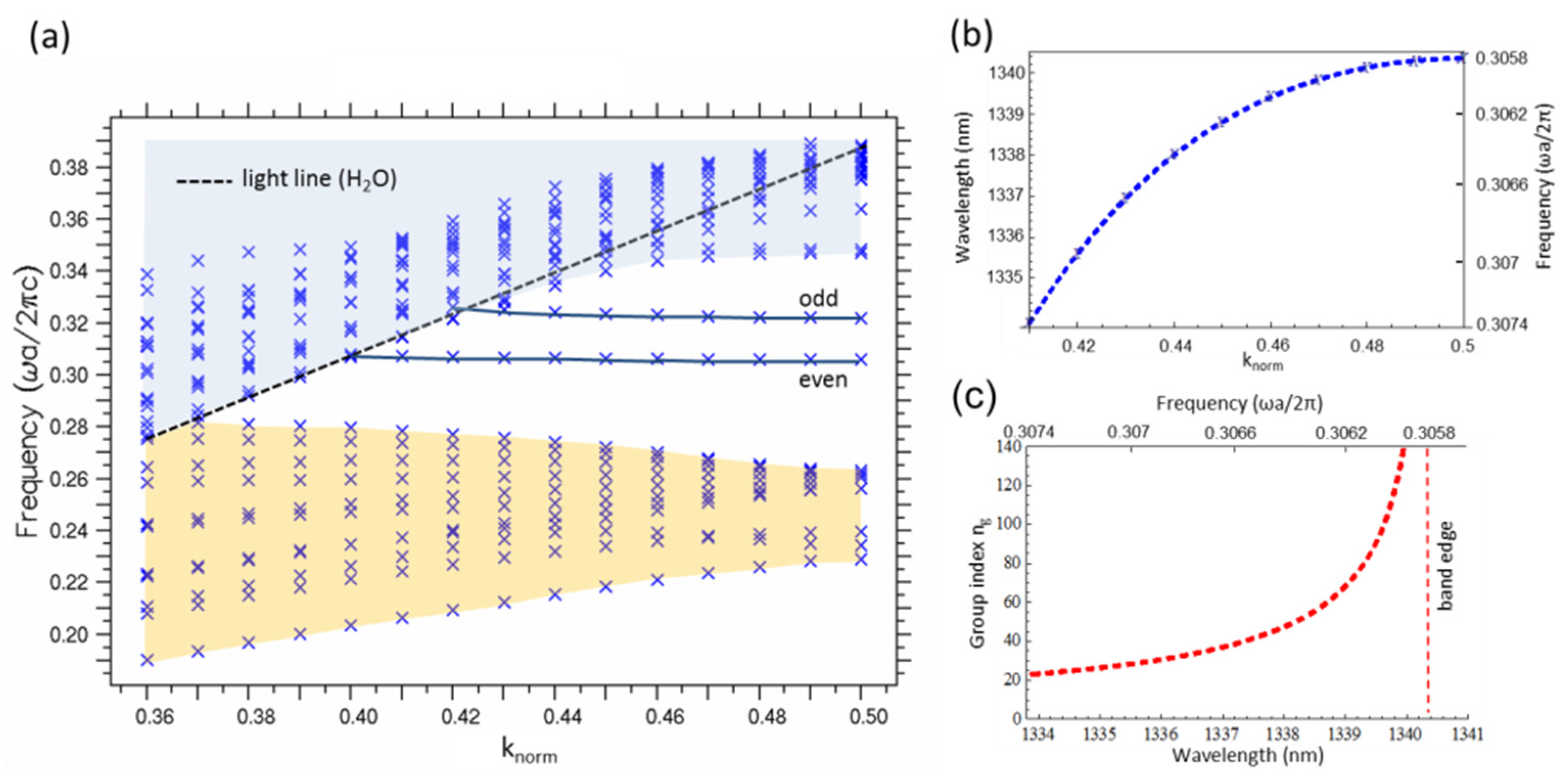
2. Sensor Concept and Photonic Crystal Defect Waveguide Design
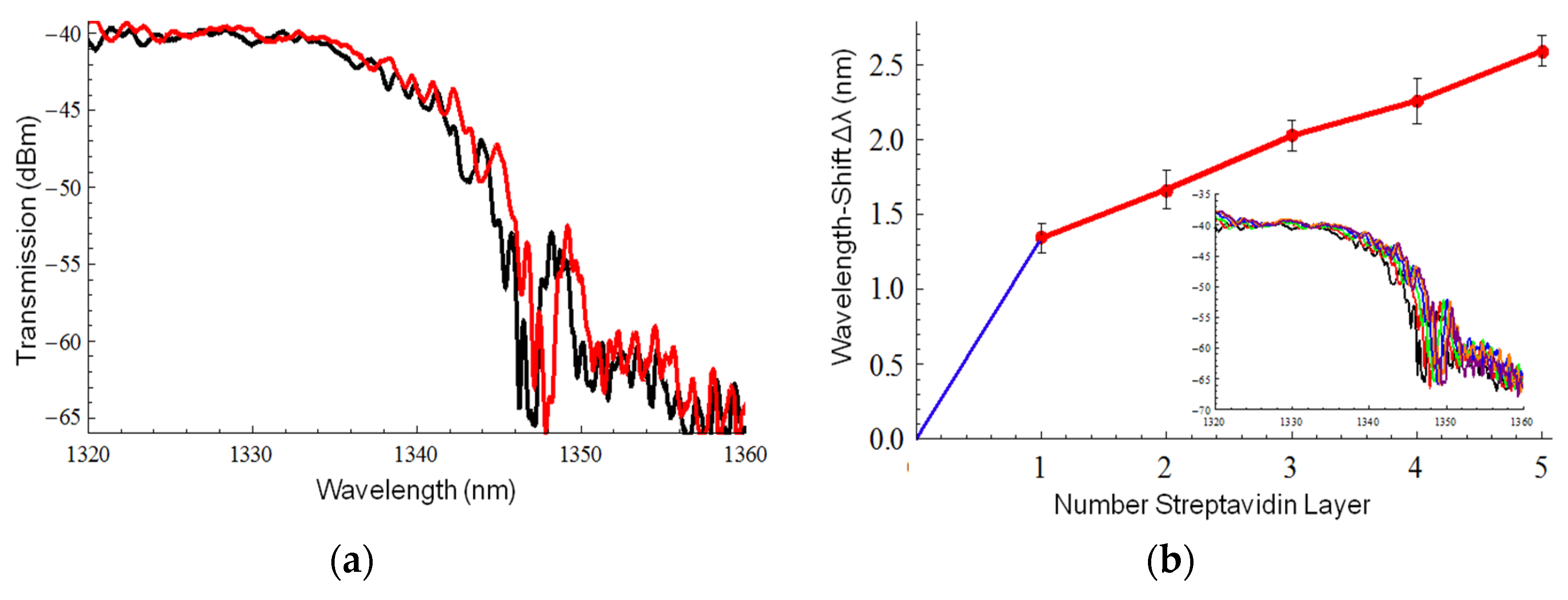
3. Measurements and Results
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Estevez, M.C.; Lechuga, L.M. Integrated optical devices for lab-on-a-chip biosensing applications. Laser Photonics Rev. 2011, 6, 463–487. [Google Scholar] [CrossRef]
- Krauss, T.F. Slow light in photonic crystal waveguides. J. Phys. D Appl. Phys. 2006, 40, 2666–2670. [Google Scholar] [CrossRef]
- Baba, T. Slow light in photonic crystals. Nat. Photonics 2008, 2, 465–473. [Google Scholar] [CrossRef]
- Barnier, D.; Cassan, E.; Le Roux, X.; Marris-Morini, D.; Vivien, L. Efficient band-edge light injection in two –dimensional planar photonic crystals using a gradual interface. Opt. Eng. 2009, 48, 070501-1–070501-3. [Google Scholar] [CrossRef][Green Version]
- Hugonin, J.P.; Lalanne, P.; White, T.P.; Krauss, T.F. Coupling into slow-mode photonic crystal waveguides. Opt. Lett. 2007, 32, 2638–2640. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.synopsys.com/optical-solutions/rsoft/passive-device-bandsolve.html (accessed on 13 November 2018).
- Melnik, E.; Muellner, P.; Bethge, O.; Bertagnolli, E.; Hainberger, R.; Laemmerhofer, M. Streptavidin binding as a model to characterize thiol–ene chemistry-based polyamine surfaces for reversible photonic protein biosensing. Chem. Commun. 2014, 50, 2424–2427. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wellenzohn, M.; Melnik, E.; Muellner, P.; O’Faolain, L.; Hainberger, R. Design of a Photonic Crystal Defect Waveguide Biosensor Operating in Aqueous Solutions at 1.34 µm. Proceedings 2018, 2, 1026. https://doi.org/10.3390/proceedings2131026
Wellenzohn M, Melnik E, Muellner P, O’Faolain L, Hainberger R. Design of a Photonic Crystal Defect Waveguide Biosensor Operating in Aqueous Solutions at 1.34 µm. Proceedings. 2018; 2(13):1026. https://doi.org/10.3390/proceedings2131026
Chicago/Turabian StyleWellenzohn, Markus, Eva Melnik, Paul Muellner, Liam O’Faolain, and Rainer Hainberger. 2018. "Design of a Photonic Crystal Defect Waveguide Biosensor Operating in Aqueous Solutions at 1.34 µm" Proceedings 2, no. 13: 1026. https://doi.org/10.3390/proceedings2131026
APA StyleWellenzohn, M., Melnik, E., Muellner, P., O’Faolain, L., & Hainberger, R. (2018). Design of a Photonic Crystal Defect Waveguide Biosensor Operating in Aqueous Solutions at 1.34 µm. Proceedings, 2(13), 1026. https://doi.org/10.3390/proceedings2131026




