Spread of Antibiotic Resistance in Aquatic Environments: E. coli as a Case Study †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sample Collection
2.3. Escherichia coli Isolation
2.4. Antibiotic Resistance
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance; Welcome Trust: London, UK, 2016; Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 19 January 2018).
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.L.; Calomiris, J.J.; Seidler, R.J. Selection of antibiotic-resistant standard plate count bacteria during water treatment. Appl. Environ. Microbiol. 1981, 44, 308–316. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC242011/ (accessed on 12 January 2018). [CrossRef] [PubMed]
- Massa, S.; Petruccioli, M.; Fanelli, M.; Gori, L. Drug resistant bacteria in non carbonated mineral waters. Microbiol. Res. 1985, 150, 403–408. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8564367 (accessed on 19 February 2018). [CrossRef]
- Xi, C.; Zhang, Y.; Marrs, C.F.; Ye, W.; Simon, C.; Foxman, B.; Nriagu, J. Prevalence of antibiotic resistance in drinking water treatment and distribution systems. Appl. Environ. Microbiol. 2011, 75, 5714–5718. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Knapp, C.W.; Beattie, T.K. Antibiotic resistant bacteria found in municipal drinking water. Environ. Process. 2016, 3, 541–552. [Google Scholar] [CrossRef]
- Papandreou, S.; Pagonopoulou, O.; Vantarakis, A.; Papapetropoulou, M. Multiantibiotic resistance of gram-negative bacteria isolated from drinking water samples in southwest Greece. J. Chemother. 2013, 12, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, M.A.; Roccia, I.L.; De Luca, M.M.; Pezzani, B.C.; Basualdo, J.A. Resistance to antibiotics in injured coliforms isolated from drinking water. Microbiol. Immunol. 2001, 45, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Pantak, S.P.; Gopal, K. Prevalence of bacterial contamination with antibiotic resistant and enterotoxigenic fecal coliforms in treated drinking water. J. Toxicol. Environ. Health A 2008, 71, 427–433. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, D.; He, S.; Ye, H.; Zhang, L.; Wen, Y.; Zhang, W.; Shu, L.; Chen, S. Prevalence of antibiotic-resistant Escherichia coli in drinking water sources in Hangzhou city. Front. Microbiol. 2017, 8, 1133. [Google Scholar] [CrossRef] [PubMed]
- Samra, Z.Q.; Naseem, M.; Khan, S.J.; Dar, N.; Athar, M.A. PCR targeting of antibiotic resistant bacteria in public drinking water of Lahore metropolitan, Pakistan. Biomed. Environ. Sci. 2009, 6, 458–463. [Google Scholar] [CrossRef]
- Patoli, A.A.; Patoli, B.B.; Mehraj, V. High prevalence of multi-drug resistant Escherichia coli in drinking water samples from Hyderabad. Gjms 2010, 8, 23–26. Available online: http://www.gjms.com.pk/files/GJMS%20Vol-8-1(5).pdf (accessed on 8 January 2018).
- Coleman, B.L.; Louie, M.; Salvadori, M.I.; McEwen, S.A.; Neumann, N.; Sibley, K.; Irwin, R.J.; Jamieson, F.B.; Daignault, D.; Majury, A.; et al. Contamination of Canadian private drinking water sources with antimicrobial resistant Escherichia coli. Water Res. 2013, 47, 3026–3036. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23548566 (accessed on 19 February 2018). [CrossRef] [PubMed]
- Amundson, D.; Lindholm, C.; Goyal, S.M.; Robinson, R.A. Microbial pollution of well water in southeastern Minnesota. J. Environ. Sci. Health A 1988, 23, 453–468. [Google Scholar] [CrossRef]
- McKeon, D.M.; Calabrese, J.P.; Bissonnette, G.K. Antibiotic resistant gram-negative bacteria in rural groundwater supplies. Water Res. 1995, 29, 1902–1908. [Google Scholar] [CrossRef]
- AbdelRahim, K.A.A.; Hassanein, A.M.; AbdelAzeiz, H.A.E.H. Prevalence, plasmids and antibiotic resistance correlation of enteric bacteria in different drinking water resources in Sohag, Egypt. Jundishapur J. Microbiol. 2015, 8, e18648. [Google Scholar] [CrossRef] [PubMed]
- Wellington, E.M.H.; Boxall, A.B.A.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect. Dis. 2013, 13, 155–165. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23347633 (accessed on 19 February 2018). [CrossRef]
- Hudzicui, J. Protocol 3189 Kirby—Bauer disk diffusion susceptibility test. ASM Sci. 2009, 1–23. Available online: http://www.asmscience.org/content/education/protocol/protocol.3189 (accessed on 18 May 2017).
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect 2014, 20, O255–O266. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24131428 (accessed on 2 December 2017). [CrossRef] [PubMed]

| Inhibition Zone mm | ||||
|---|---|---|---|---|
| Antibiotic | Concentration | Resistant | Intermediate | Sensitive |
| Amoxicillin AMX | 25 μg | ≤1.3 | 1.4–1.6 | ≥1.7 |
| Cefaclor CEC | 30 μg | ≤1.4 | 1.5–1.7 | ≥1.8 |
| Norfloxacin NXN | 10 μg | ≤2.0 | 2.1–2.4 | 2.5–3.1 |
| Ciprofloxacin CIP | 5 μg | ≤2.4 | 2.5–2.7 | 2.8–3.5 |
| Levofloxacin LVX | 5 μg | ≤.5 | 2.6–2.8 | 2.9–3.6 |
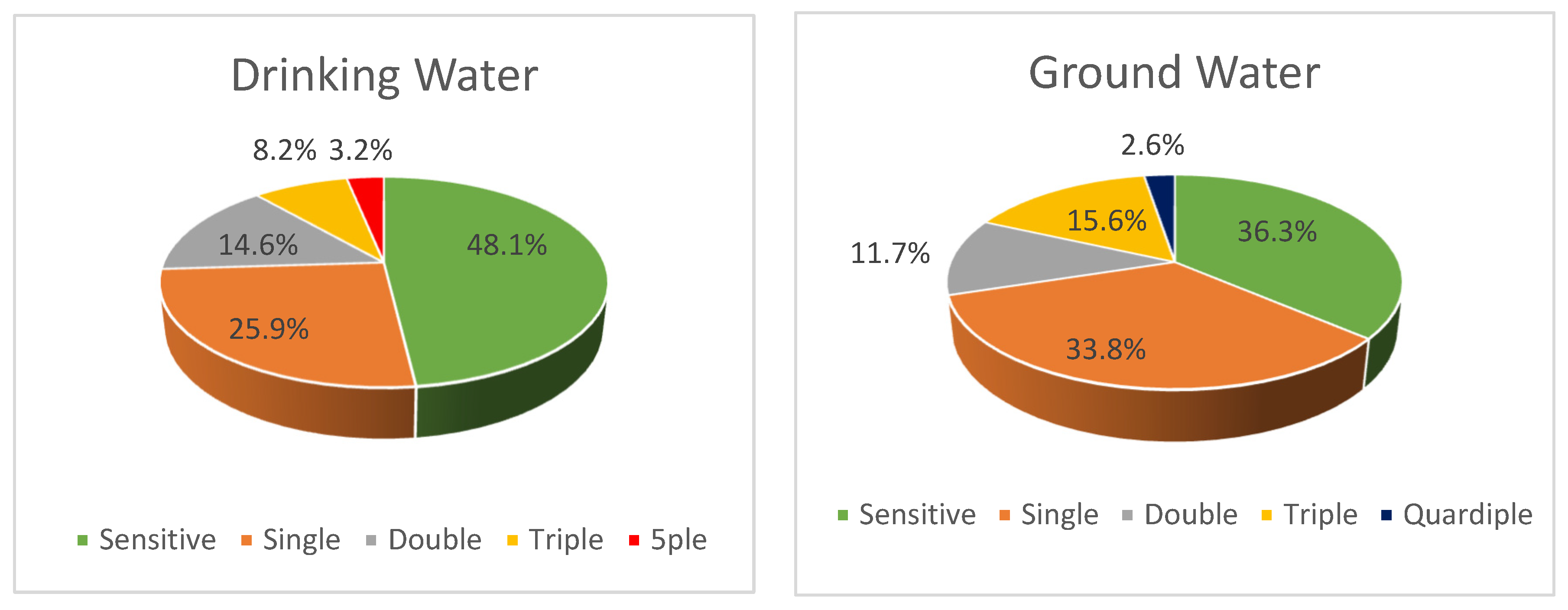
| Resistance to: | Drinking Water | Ground Water | Total |
|---|---|---|---|
| Strains (%) 1 | Strains (%) 1 | Strains (%) 1 | |
| 1 antibiotic | 41 (25.9) | 26 (33.8) | 67 (28.5) |
| 2 antibiotics | 23 (14.6) | 9 (11.7) | 32 (13.6) |
| 3 antibiotics | 11 (7.0) | 12 (15.6) | 23 (9.8) |
| 4 antibiotics | - | 2 (2.6) | 2 (0.8) |
| 5 antibiotics | 5 (3.1) | - | 5 (2.1) |
| Total strains | 80 (50.6) | 49 (63.7) | 129 (54.9) |
| AMX Number 1—% | CEC Number 1—% | NXN Number 1—% | CIP Number 1—% | LVX Number 1—% | |
|---|---|---|---|---|---|
| Drinking | 54–34.2% | 29–18.4% | 9–5.7% | 11–7.0% | 42–26.6% |
| Ground | 36–46.8% | 14–18.2% | 4–5.2% | 9–11.7% | 25–32.5% |
| Total | 90–38.3% | 43–18.3% | 13–5.5% | 20–8.5% | 67–28.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efstratiou, M.A.; Bountouni, M.; Kefalas, E. Spread of Antibiotic Resistance in Aquatic Environments: E. coli as a Case Study. Proceedings 2018, 2, 693. https://doi.org/10.3390/proceedings2110693
Efstratiou MA, Bountouni M, Kefalas E. Spread of Antibiotic Resistance in Aquatic Environments: E. coli as a Case Study. Proceedings. 2018; 2(11):693. https://doi.org/10.3390/proceedings2110693
Chicago/Turabian StyleEfstratiou, Maria Adamantia, Marina Bountouni, and Efthimios Kefalas. 2018. "Spread of Antibiotic Resistance in Aquatic Environments: E. coli as a Case Study" Proceedings 2, no. 11: 693. https://doi.org/10.3390/proceedings2110693
APA StyleEfstratiou, M. A., Bountouni, M., & Kefalas, E. (2018). Spread of Antibiotic Resistance in Aquatic Environments: E. coli as a Case Study. Proceedings, 2(11), 693. https://doi.org/10.3390/proceedings2110693




