Modelling Dimethoate Removal by Fenton-Like Process Using Response Surface Methodology †
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Experimental Procedure
2.3. Analytical Methods
2.4. Experimental Design, Data Analysis and Process Optimization
3. Results and Discussion
3.1. Fitting the RSM to Significant Independent Variables
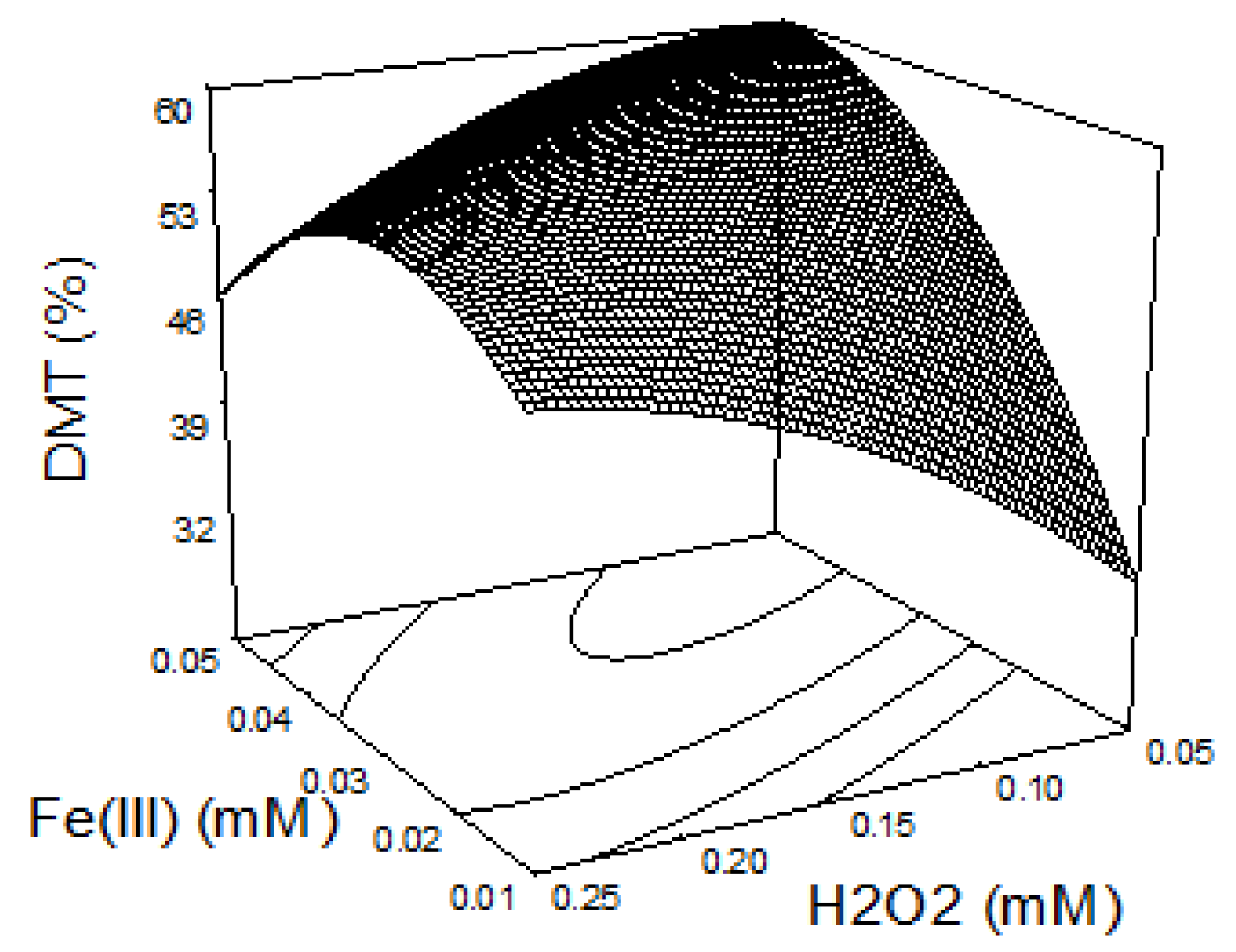
3.2. DMT Oxidation
3.3. DMT Mineralization
4. Conclusions
Acknowledgments
References
- Domínguez, C.; García, J.; Pedraz, M.A.; Torres, A.; Galán, M.A. Photocatalytic oxidation of organic pollutants in water. Catal. Today 1998, 40, 85–101. [Google Scholar] [CrossRef]
- Evgenidou, E.; Fytianos, K.; Poulios, I. Photocatalytic oxidation of dimethoate in aqueous solutions. J. Photochem. Photobiol. A Chem. 2005, 175, 29–38. [Google Scholar] [CrossRef]
- Aimer, Y.; Benali, O.; Salghi, R.; Latapie, L.; GroenenSerrano, K. Removal of Pesticides by Electrochemical Oxidation using a Boron Doped Diamond Anode. J. Mater. Environ. Sci. JMES 2017, 8, 777–783. [Google Scholar]
- Meijers, R.T.; Oderwald-Muller, E.; Nuhn, P.A.N.M.; Kruithof, J.C. Degradation of Pesticides by Ozonation and Advanced Oxidation. Ozone Sci. Eng. 1995, 17, 673–686. [Google Scholar] [CrossRef]
- Ormad, M.P.; Miguel, N.; Lanao, M.; Mosteo, R.; Ovelleiro, J.L. Effect of application of ozon combined with hydrogen peroxide and titanium dioxide in the removal of pesticides from water. Ozone Sci. Eng. 2010, 32, 25–32. [Google Scholar] [CrossRef]
- Quiroz, M.A.; Martínez-Huitle, C.A.; Bandala, E.R. Advanced oxidation processes (AOPs) for removal of pesticides from aqueous media. In Pesticides—Formulations, Effects; Stoytcheva, M., Ed.; InTech Open Access Publisher: Rjeka, Cratia, 2011; pp. 685–730. [Google Scholar]
- Park, J.H.; Cho, I.H.; Chang, S.W. Comparison of fenton and photo-fenton processes for livestock wastewater treatment. J. Environ. Sci. Health Part B 2006, 41, 109–120. [Google Scholar] [CrossRef]
- Kuo, W.G. Decolorizing dye wastewater with Fenton’s reagent. Water Res. 1992, 26, 881–886. [Google Scholar] [CrossRef]
- Yi, X.; Sun, L.; Yu, J.; Wang, S.; Yu, S. Oxidation of acetyl-pyrimidine wastewater by Fenton process. Water Sci. Technol. 2010, 62, 2630–2636. [Google Scholar] [CrossRef][Green Version]
- Mason, R.L.; Gunst, R.F.; Hess, J.L. Statistical Design and Analysis of Experiments, Eighth Applications to Engineering and Science, 2nd ed.; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Wu, Y.; Zhou, S.; Qin, F.; Ye, X.; Zheng, K. Modelling physical and oxidative removal properties of Fenton process for treatment of landfill leachate using response surface methodology (RSM). J. Hazard. Mater. 2010, 180, 456–465. [Google Scholar] [CrossRef]
- Segura, C.; Zaror, C.; Mansilla, H.D.; Mondaca, M.A. Imidacloprid oxidation by photo-fenton reaction. J. Hazard. Mater. 2008, 150, 679–686. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Tureli, G.; Olmez-Hanci, T. Treatment of azo dye production wastewaters using Photo-Fenton like advanced oxidation processes: Optimization by response surface methodology. J. Photochem. Photobiol. A 2009, 202, 142–153. [Google Scholar] [CrossRef]
- Almeida, L.C.; Garcia-Segura, S.; Bocchi, N.; Brillas, E. Solar photoelectro-fenton degradation of paracetamol using a flow plant with a Pt/air-diffusion cell coupled with a compound parabolic collector: Process optimization by response surface methodology. Appl. Catal. B Environ. 2011, 103, 21–30. [Google Scholar] [CrossRef]
- Aybastier, O.; Demir, C. Optimization of immobilization conditions of thermomyceslanuginosus lipase on styrene-divinylbenzene copolymer using response surface methodology. J. Mol. Catal. BEnzym. 2010, 63, 170–178. [Google Scholar] [CrossRef]
- Sanchez-Lafuente, C.; Furlanetto, S.; Fernandez-Arevalo, M. Didanosine extended-release matrix tablets: Optimization of formulation variables using statistical experimental design. Int. J. Pharm. 2002, 237, 107–118. [Google Scholar] [CrossRef]
- Üstün, G.E.; Akal Solmaz, S.K.; Morsunbul, T.; Azak, H.S. Advanced oxidation and mineralization of 3-indole butyric acid (IBA) by fenton and fenton-like processes. J. Hazard. Mater. 2010, 180, 508–513. [Google Scholar] [CrossRef]
- Madhavana, J.; Kumar, S.S.P.; Anandan, S.; Grieser, F.; Ashokkumar, M. Sonophotocatalytic degradation of monocrotophos using TiO2 and Fe3+. J. Hazard. Mater. 2010, 177, 944–949. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Washington, DC, USA; Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
- YaliliKilic, M.; Akal Solmaz, S.K.; Üstün, G.E.; Azak, S.H. Investigation of oxidation and mineralization of the pesticide dimethoate, an endocrine disruptor, using response surface methodology and Fe+2 process. In Proceedings of the Sixth International Conference on Environmental Management, Engineering, Planning & Economics, Thessaloniki, Greece, 25–30 June2017. [Google Scholar]
- Tamimi, M.; Qourzal, S.; Barka, N.; Assabbane, A.; Ait-Ichou, Y. Methomyl degradation in aqueous solutions by Fenton’s reagent and the photo-fenton system. Sep. Purif. Technol. 2008, 61, 103–108. [Google Scholar] [CrossRef]
- Lucas, M.S.; Jose, A. Peres Decolorization of the azo dye Reactive Black 5 by Fenton and photo-Fenton oxidation. Dyes Pigments 2006, 71, 236–244. [Google Scholar] [CrossRef]
- Chen, R.Z.; Pignatello, J.J. Role of quinone intermediates as election shuttle in Fenton and photoassisted Fenton oxidation of aromatic compounds. Environ. Sci. Technol. 1997, 312, 399–2406. [Google Scholar]
- Sun, J.H.; Sun, S.P.; Fan, M.H.; Guo, H.Q.; Qiao, L.P.; Sun, R.X. A kinetic study on the degradation of P-nitroaniline by fenton oxidation process. J. Hazard. Mater. 2007, 148, 172–177. [Google Scholar] [CrossRef]
- Tony, M.A.; Bredi, Z. Experimental design of photo-Fenton reactions for the treatment of car wash wastewater effluents by response surface methodological analysis. Adv. Environ. Chem. 2014, 2014, 958134. [Google Scholar] [CrossRef]
- Ghaly, M.Y.; Hartel, G.; Mayer, R.; Haseneder, R. Photochemical oxidationof P-chlorophenol by UV/H2O2 and photo-Fenton process. A comparative study. Waste Manag. 2001, 21, 41–47. [Google Scholar] [CrossRef]
- Rodriguez, M.; Malato, S.; Pulgarin, C.; Contreras, S.; Curco, D.; Gimenez, J.; Esplugas, S. Optimizing the solar photo-fenton process in the treatment of contaminated water, determination of intrinsic kinetic constants for scale-up. Solar Energy 2005, 79, 360–368. [Google Scholar] [CrossRef]
- Ying-Shih, M.; Chi-Fanga, S.; Jih-Gaw, L. Degradation of Carbofuran in aqueous solution by ultrasound and Fenton processes: Effect of system parameters and kinetic study. J.Hazard. Mater. 2010, 178, 320–325. [Google Scholar]
- Masomboon, N.; Chen, C.; Anotai, J.; Lu, M. A statistical experimental design to determine O-toluidine degradation by the photo-Fenton process. Chem. Eng. J. 2010, 159, 116–122. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalbur, B.E.; Teksoy, A.; Solmaz, S.K.A.; Azak, H.S. Modelling Dimethoate Removal by Fenton-Like Process Using Response Surface Methodology. Proceedings 2018, 2, 649. https://doi.org/10.3390/proceedings2110649
Nalbur BE, Teksoy A, Solmaz SKA, Azak HS. Modelling Dimethoate Removal by Fenton-Like Process Using Response Surface Methodology. Proceedings. 2018; 2(11):649. https://doi.org/10.3390/proceedings2110649
Chicago/Turabian StyleNalbur, Berrak Erol, Arzu Teksoy, Seval Kutlu Akal Solmaz, and Hilal Safiye Azak. 2018. "Modelling Dimethoate Removal by Fenton-Like Process Using Response Surface Methodology" Proceedings 2, no. 11: 649. https://doi.org/10.3390/proceedings2110649
APA StyleNalbur, B. E., Teksoy, A., Solmaz, S. K. A., & Azak, H. S. (2018). Modelling Dimethoate Removal by Fenton-Like Process Using Response Surface Methodology. Proceedings, 2(11), 649. https://doi.org/10.3390/proceedings2110649




