Abstract
The article investigated the effects of ultrasound pretreatment on biological treatment of landfill leachate. Leachates with and without conditioning were combined with municipal wastewater at different ratios. The study showed that the implementation of a pretreatment step prior to biological treatment not only results in higher pollutant removal efficiency but may also allow for an increased leachate volume share in the influent stream entering the reactor by up to 20% (quality of effluents meets national regulation requirements) which in scenarios without pretreatment cannot exceed 5% due to poor quality of the effluents.
Keywords:
leachate; ultrasound; SBR; co-treatment; municipal wastewater; leachate pretreatment; mixing ratio 1. Introduction
One of the most serious environmental problems associated with disposal of municipal solid waste is the generation of landfill leachate. Its composition and amount depends on many factors some of them being: (a) the type and amount of waste deposited and the degree of their grinding; (b) climate conditions, including the volume and frequency of precipitation, air humidity, as well as evaporation rate; (c) age of the landfill; (d) storing technology, and therefore the degree of waste compaction as well as the method of sealing the landfill; (e) humidity of waste; (f) volume of precipitation infiltrating through the bed; (g) geomorphology and topography of the area where the landfill is located; (h) the lifetime of the landfill; (i) reclamation and the type of vegetation covering the top of the landfill after its shutdown; (j) the runoff direction of rainwater and snowmelt [1,2,3].
High concentrations of toxic pollutants (such as heavy metals, ammonia) and refractory compounds as well as seasonal variation in the composition and amount of leachate result in difficulties with its treatment [4]. Despite that, there are several known methods of leachate treatment, which incorporate various physical, chemical, and biological processes [5]. Based on the nature of the incorporated processes methods of leachate treatment can be grouped as conventional and advanced treatments, as done by Renou et al. [4]. The main conventional landfill leachate treatments include: (a) biodegradation (via aerobic and/or anaerobic processes); (b) chemical and physical methods such as: adsorption, coagulation, sedimentation/flotation, chemical oxidation, coagulation/flocculation, chemical precipitation as well as air stripping; (c) co-treatment of leachate with other wastewaters such as municipal in wastewater treatment plants. Whereas membrane technologies as well as technologies based on advanced oxidation processes (AOPs) are regarded as potential alternatives for leachate treatment (advanced treatments).
Selection of the appropriate leachate treatment method is based on its composition and properties (mainly physicochemical characteristics as well as its age) [4,5,6]. Young leachate due to high BOD5/COD ratio can be effectively treated using biological methods [7]. While for mature or stabilized landfill leachates, where BOD5/COD ratios are below 0.1, they are deemed ineffective [8]. Therefore, this type of leachate requires either the use of alternative treatment processes or increasing its susceptibility to biodegradation via application of pretreatment methods making biological treatment a viable option [6].
Due to low operating cost and easy maintenance co-treatment of landfill leachate with readily biodegradable wastewater (for example municipal wastewater) seems a promising approach. So far, this solution has been successfully used for the treatment of young and intermediate leachates (mostly at a volumetric ratio of up to 10%) [4,9]. However, this poses a risk of disrupting the operation of biological reactors and is the main argument against the application of this solution. Due to high content of non-biodegradable organics and inorganics as well as toxic compounds, the introduction of leachate to a biological reactor may result in the inhibition of the activated sludge treatment process and consequently lead to reduced treatment efficiency and increased pollutant concentration in the effluent [4,7]. For this reason, pre-treatment of leachate prior to its joint biological treatment with municipal wastewater seems justified. However, publications on this subject are very limited. Furthermore, very few systematic studies are available for mature leachate [6,10,11,12]. For instance, Wang et al. [10] demonstrated that using a combination of coagulation, Fenton oxidation and biological aerated filter process COD may be reduced to 75 mg/L. While Guo et al. [12] evaluated the feasibility of a mature leachate treatment consisting of a combination of physicochemical (air stripping, Fenton, coagulation) and biological processes (sequencing batch reactor (SBR)). The authors found that the solution was an attractive alternative when dealing with high-strength wastewater, allowing for an over 95% removal of COD, BOD5 and ammonium nitrogen. However, there is no information regarding how pre-treatment of mature leachate with an ultrasound field affects its biological treatment. Therefore, the aim of this investigation was to determine the effects of low energy ultrasound irradiation on sequencing batch reactor (SBR) treatment of landfill leachate. The effect of the volume ratio of leachate (with and without pre-treatment) on the removal efficiency of COD, BOD5 and ammonium nitrogen was also evaluated in this paper. Additionally, special attention was paid to the influence of pre-treatment methods on the condition of activated sludge by assessing the impact of the volume ratio of leachate (with and without pre-treatment) on dehydrogenase activity (DHA) as well as respiratory activity of the activated sludge.
2. Materials and Methods
2.1. Materials
Leachate for all experiments was obtained from a sanitary landfill site in southern Poland (Silesian Region). Raw domestic watewater as well as activated sludge (for biochemical tests) were collected from a municipal wastewater treatment plant (WWTP), with a treatment capacity of 314 835 population equivalent with an average wastewater flow rate of 90,000 m3/d.
Composition of leachate is shown in Table A1 (Appendix A, Table A1). Taking into account the high pH values (>8.1) as well as high concentration of ammonium ions and low BOD5/COD ratio (0.11), the leachate can be classified as stabilized or mature.
2.2. Experimental Procedure
The experiment was divided into two stages. During the first stage, the optimum time for the solubilization of organic matter in the leachate samples was investigated using the UD VCX 1500 disintegrator with a field frequency of 20 kHz and an amplitude of 12 µm. The amplitude of the ultrasonic field was selected based on the results from the Authors previous research [13]. Additionally, in order to determine the toxicity of landfill leachates on the activated sludge microorganisms, dehydrogenase activity (DHA) as well as respiratory activity of the activated sludge was measured. For the purposes mentioned above, the sample (activated sludge collected from the WWTP) was prepared by executing the following steps: (1) washing/flushing with tap water; (2) removal of thicker slurry, (3) 24 h aeration. After which the sample underwent biochemical tests. The trials were performed for activated sludge without leachate (reference sample—the leachate addition impact on the investigated indicators has been evaluated in relation to the results obtained for this sample—percentage increment) as well as mixtures of activated sludge with leachate (with and without pretreatment). The percentage increment of dehydrogenase activity (DHA) as well as respiratory activity of the activated sludge was calculated using the following equations:
Percentage increment of the parameter = ((value for mixture-value for reference sample)/value for reference sample) × 100%
The optimal conditioning time was selected based on the values of COD, BOD5/COD ratio as well as results of biochemical activity tests performed for the activated sludge. In the 2nd stage, two identical laboratory-scale SBRs with a working volume of 3 L were used for the examination of leachate ultrasound pretreatment on biological treatment efficiency. The SBR systems were operated at feeding condition of leachate dilution of up to 45% by volume with raw domestic wastewater and sludge concentration of 4 g/L. Samples were withdrawn from the reactor at the beginning and at the end of each cycle for analysis. The scheme of the experiment is shown in Figure 1.
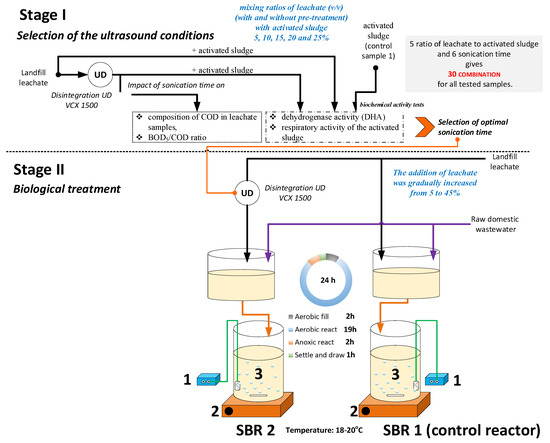
Figure 1.
Experimental set-up and operational modes of the SBR; where: 1—Air pomp, 2—Magnetic stirrer, 3—SBR.
2.3. Sample Analyses
In the study, the following parameters were investigated: pH value, Kjeldahl nitrogen (TKN), ammonium nitrogen (N–NH4+), chloride, total suspended solids (TSS), nitrate (NO3−), nitrite (NO2−), alkalinity, phosphates (PO43—P), phosphate total, chemical oxygen demand (COD), biochemical oxygen demand (BOD5). Additionally, in the case of COD (CODtot.) its composition in leachate samples was size-fractioned into the following fractions: suspended fractions (CODsups.) (>4.4 μm); dissolved fractions (CODdis.) (<0.45 μm) and colloid fractions (CODcol.) based on the molecular weight distribution during filtration through a membrane filter. With the exception of the last fraction which was calculated using the following equation:
CODcol. = CODtot. − CODsusp. − CODdis.
All of the mentioned analyses were performed according to the APHA Standard Methods for the Examination of Water and Wastewater [14].
The respiratory activity of the activated sludge was determined based on the specific oxygen uptake rate (SOUR). This measurement was performed according to the US. Environmental Protection Agency method (EPA 1863) [15]. The TTC test was used to determine the enzymatic activity (DHA) of the activated sludge. The measurement of DHA was performed in accordance with [16].
The statistical analyses of the obtained results were carried out using STATISTICA software (STATISTICA 12 PL StatSoft, Inc., Cracow, Poland). One-way analyses of variance (ANOVA) was used to determine the main effect of ultrasound sonication time on selected parameters. While, in the case of biochemical test, factorial ANOVA was performed. Assumption for variances in the form of its homogeneity was checked using Levene’s test. In the case of data which failed the ANOVA assumptions it was analyzed via the Kruskal–Wallis test. For statistically significant data, Tuckey’s test was performed. The statistical estimation was done with at least three replications for each combination of the nominal variables.
3. Results and Discussion
3.1. First Stage
Generally, as depicted in Table A2, the BOD5/COD ratio increased with the gradual increase of sonication time. However, for the first of the tested sonication times this ratio was insignificantly higher than for the non-conditioned sample and ranged from 0.14 to 0.18. Extending the sonication time to 3 min caused an increment of this parameter of approximately 273% (from 0.11 to 0.3). However, further extension of the ultrasound sonication time did not have a statistically significant effect on the value of BOD5/COD. As shown in the extended review written by Renou et al. [4], the positive impact of advanced oxidation processes (AOPs) on BOD5/COD ratio has been reported in many studies. For example, Chou et al. [17] reported that BOD5/COD ratio increased with elongation of microwave oxidation time from 0.05 for the control sample to 0.12 for the longest time which was investigated by these Authors. Moreover Lopez et al. [18] observed an increase of this ratio from 0.2 (the initial value) up to 0.5, after pretreating the leachate using the Fenton process. Cortez at al. [19] noted the increase of this ratio from 0.01 to 0.17 after the O3/H2O2 process. Hu et al. [20] also observed an increase of the 5-day biochemical oxygen demand (BOD5) to COD ratio from 0.17 to 0.60, when Fenton reagent, UV–Fenton or UV–H2O2, were used to treat mature landfill leachate.
The obtained results (Table A2) also showed that pretreatment had the slightest impact on the CODsusp. and CODcol. concentrations. Both indicators decreased with the increase of sonication time. The lowest average for both fractions of COD (35,223 mg/L) were obtained for sonication time of 3 min. An opposite trend was observed for pH, which decreased along with the elongation of the sonication time. In comparison to the results obtained for the control sample, the longest sonication time resulted in a pH value decrease of 22% (from 8.3 to 6.5).
Results of biochemical tests are shown in Figure 2 and Figure 3. The values of both parameters strongly depended on the share of leachates in the mixtures regardless of the method of preparing the leachate (with or without pretreatment). For both parameters, the highest values were observed for samples containing 15% of leachate. For activated sludge with non-conditioned leachate the SOUR was approximately 22 mgO2/g·h and thus was 250% higher than the values noted for the reference sample (an average for 30 samples: 6.28 ± 0.43 mgO2/g·h). After increasing the share of leachate in the mixture, the percentage increment of SOUR in comparison to the reference sample decreased to 120%, while for the highest volumetric ratio of leachate to wastewater, it did not exceed 8%. A similar trend was observed for activated sludge with pretreated leachate. However, the obtained values were significantly higher than those achieved for activated sludge with non-conditioned leachate. Significant differences in SOUR occurred in the mixtures containing 20% and 25% of leachate which was sonicated for 5, 10 and 15 min. In the case of these samples, the percentage increment of SOUR values was about 200% and approximately 50%, respectively.
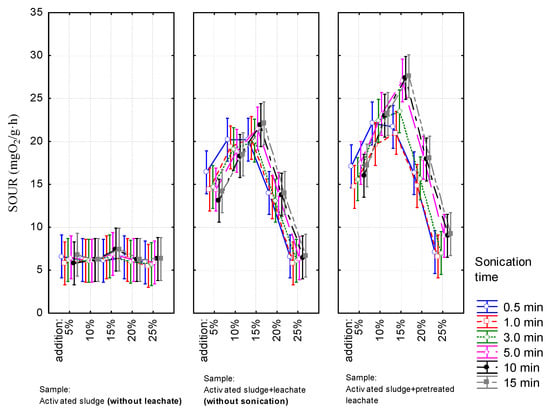
Figure 2.
The SOUR profile at different the volumetric ratio of leachate in mixture.
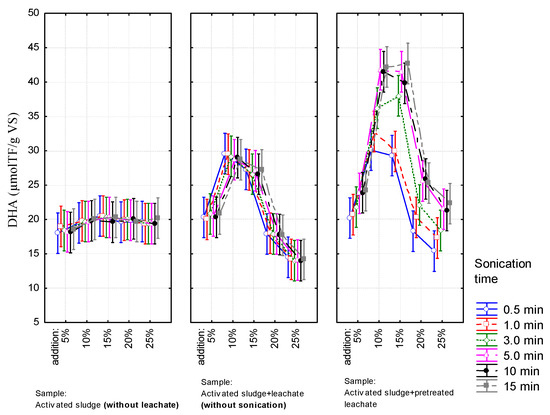
Figure 3.
The DHA activity profile at different the volumetric ratio of leachate in mixture.
Similar trends like in the case of SOUR were observed for the DHA activity. With prolongation of sonication time, the difference in DHA values between samples increased (with and without pretreatment). The highest percentage increment of dehydrogenase activity (approx. 110% in comparison to the reference sample) was observed for the samples containing 10% and 15% of leachate in the mixture at an ultrasound field exposure duration of above 5 min. These values were significantly higher than those obtained for the reference sample as well as activated sludge with non-conditioned leachate. It should be emphasized that for the trials for leachate without conditioning, the DHA activity was lower than in the reference sample. This proves the positive effect of conditioning on the condition of activated sludge.
Factorial ANOVA (F-values for selected parameters), confirmed the above observations namely that the volumetric ratio of leachate in the mixture had the greatest impact on values of both biochemical indicators (F = 210 and F = 151 for oxygen consumption rate and DHA activity respectively, for all p < 0.05), while the method of leachate preparation affects them to a much lesser extent (F = 51 and F = 128 for rate of oxygen consumption and DHA activity, respectively for all p < 0.05). In the case of activated sludge with pretreated leachate, the sonication time had no effect on SOUR (F = 0.8, p = 0.55). It did impact DHA activity (F = 2.76, p = 0.02) however, as shown by the post-hoc test any sonication executed beyond 3 min. does not have a statistically significant effect on DHA activity.
Based on the obtained results sonication time equal to 3 min was selected for further studies.
3.2. Second Stage—Biological Treatment
As depicted in Figure 4 in both SBRs the removal efficiency of COD, BOD as well as ammonium nitrogen decreased with the increase of leachate in the influent. However, regardless of the ratio of leachate in the effluent (%, v/v), the treatment efficiency was higher for SBR2 (pretreated leachate) than SBR1 (control). Thus, the execution of a pretreatment step prior to biological treatment allows to reduce the negative impact of the leachate on the removal degree for the tested parameters. This result is in agreement with the findings of El-Gohary and Kamel [7]. The mentioned Authors observed low COD and BOD removal values, 37.1 and 30.3%, respectively for intermediate leachate (BOD/COD ratio was in the range of 0.33–0.45) mixed with municipal wastewater in a ratio of 1:1. However, after pretreating the leachate via air stripping, they observed significantly increased COD and BOD removal values of up to 64.4 and 67.2%, respectively.
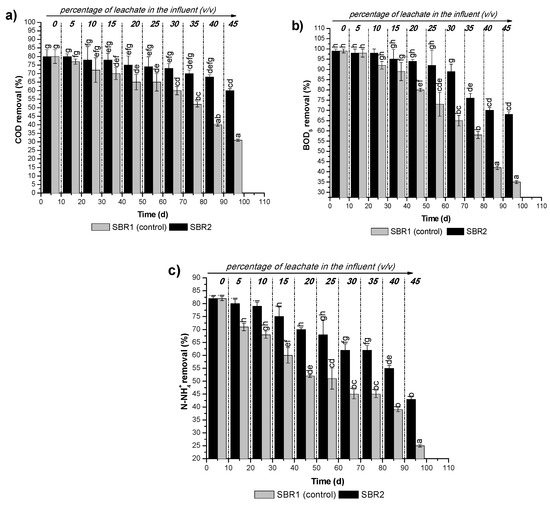
Figure 4.
Variation of treatment efficiency during the experiment: (a) COD removal; (b) BOD5 removal; (c) N–NH4+ removal.
Up to date, as shown in some studies [4,9,21], co-treatment of leachate with domestic wastewater without adverse impact on the removal efficiency of pollutants is possible if the share of the leachate in the effluent does not exceed 10% (Table A3). However, as the results obtained in this study show, the implementation of a pretreatment step before biological treatment may lead to an increase in the volume of leachate in the effluent stream entering the sewage treatment plant by up to 20%. For such leachate volumetric ratio, the removal efficiencies are within the acceptable ranges defined by Polish legislation [22], (Table A4). Without conditioning, the share of leachate in the mixture cannot be higher than 5%. If this condition was not met, the quality of effluents was below country regulation values.
4. Conclusions
The study revealed that sonication of landfill leachates increased leachate biodegradability and reduced its toxicity to microorganisms of the activated sludge. Thus, the preliminary leachate conditioning not only positively affected the condition of sewage sludge but also enhanced its treatment efficiency. The volumetric ratio of leachate in the mixture had the highest impact on the obtained results. The use of an ultrasound field allows for executing the treatment process with leachate addition higher than 10% which is the threshold limit currently stated in literature.
Author Contributions
The experiments were designed and carried out by E.N. who in partnership with Grosser analyzed the acquired data, while M.M. contributed reagents and materials. A.G. carried out statistical analyses of the results and wrote the paper in consultation with P.C. who also performed linguistic and translation revisions.
Funding
The study was supported by the BS/PB-401-301/13.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Characteristic of wastewater used in this study.
Table A1.
Characteristic of wastewater used in this study.
| Parameter | Unit | Leachate | Municipal Wastewater |
|---|---|---|---|
| pH | - | 8.1–8.5 | 6.5–7.9 |
| alkalinity | mgCaCO3/L | 15,000–12,300 | |
| TKN | mg/L | 820–1100 | 30–72 |
| NH4+ | mg/L | 750–990 | 23–60 |
| NO2− | mg/L | 25–67 | bdl |
| NO3− | mg/L | 16–28 | 0.0–1.63 |
| PO43−–P | mg/L | 11–26 | 3.5–4.2 |
| P total | mg/L | 14.1–16.7 | 6.5–7.0 |
| CODtot. | mgO2/L | 3600–4500 | 250–460 |
| BOD5 | mgO2/L | 380–530 | 120–390 |
| TSS | mg/L | 615–730 | 48–130 |
| chloride | mg/L | 1350–3200 | 51–110 |
bdl—below detection limit.

Table A2.
Impact of sonication time on the biodegradability of organic compounds (with statistical analyses-letters denote different statistical groups).
Table A2.
Impact of sonication time on the biodegradability of organic compounds (with statistical analyses-letters denote different statistical groups).
| Sonication Time (min.) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 3.0 | 5.0 | 10.0 | 15.0 | ||
| CODtot. | mgO2/L | 4650 ± 419 | 4426 ± 487 | 4231±508 | 3886 ± 311 | 3890 ± 195 | 3875 ± 271 | 3860 ± 301 |
| CODsusp. | mgO2/L | 2075 ± 125c | 1848 ± 240ab | 1685 ± 152ab | 1470 ± 103a | 1475 ± 89a | 1460 ± 117a | 1487 ± 134a |
| CODcol. | mgO2/L | 865 ± 87d | 715 ± 79cd | 617 ± 74bc | 404 ± 32a | 399 ± 20a | 397 ± 28a | 480 ± 38ab |
| CODdis. | mgO2/L | 1710 ± 137 | 1863 ± 205 | 1930 ± 232 | 2012 ± 161 | 2016 ± 101 | 2018 ± 141 | 1893 ± 151 |
| BOD5 | mgO2/L | 500 ± 45a | 691 ± 76a | 980 ± 118b | 1171 ± 94bc | 1172 ± 59bc | 1150 ± 81bc | 1225 ± 98c |
| pH | - | 8.3 ± 0.08f | 8 ± 0.08e | 7.5 ± 0.08d | 6.6 ± 0.07c | 6.5 ± 0.07ab | 6.4 ± 0.06a | 6.5 ± 0.07ab |
| BOD5/COD | - | 0.11 ± 0.01a | 0.16 ± 0.02a | 0.23 ± 0.03b | 0.30 ± 0.02c | 0.30 ± 0.02c | 0.30 ± 0.02c | 0.33 ± 0.03c |

Table A3.
Removal efficiency of selected indicators in co-treatment of leachate with wastewater.
Table A3.
Removal efficiency of selected indicators in co-treatment of leachate with wastewater.
| Type of Pretreatment and/or +Additional Process | COD (mg/L) | BOD/COD | Kind of Reactor | Volume of Reactor (L) | Temp. | Addition of Leachate (% v/v) | Removal (%) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| BOD5 | COD | NH4+ | ||||||||
| - | 1090 | 0.4 | SBR | - | 20 | 10 | 95 | - | - | [4] |
| - | 10,750 | 0.59 | SCFB | 2 | - | 6.7 | - | 89 | - | [23] |
| +PAC | SCFB | 6.7 | - | 88 | - | |||||
| - | SCFB | 13.3 | - | 78 | - | |||||
| +PAC | SCFB | 13.3 | - | 82 | - | |||||
| - | CF | 3.6 settling tank, 2.5 aeration tank | 6.7 | - | 87 | - | ||||
| +PAC | CF | 6.7 | - | 93 | - | |||||
| - | CF | 13.3 | - | 81–89 | - | |||||
| +PAC | CF | 13.3 | - | - | - | |||||
| with 4000 mg/L FeSO4 and an anionic polyelectrolyte of type SF-380 before mixing with domestic wastewater | 37,024 | 0.42 | AS | 2 | 22 ± 2 | 2–10 | - | 82–87 | - | [24] |
| - | 2431 | 0.21 | 5–20 | 16–74 | ||||||
| Without air striping/with air striping | 2366 | 0.12 | SBR | 3 | - | 2.5 | - | 87/87 | 32.1/24 | [6] |
| 5 | 80/80 | 41.1/26.2 | ||||||||
| 10 | 63/63 | 54.6/35.5 | ||||||||
| - | 10,250–16,250 | 0.33–0.45 | - | 2 | 25 | 50 | 30.3 | 37.1 | - | [7] |
| air striping | 64.4 | 67.2 | 89.3 | |||||||
| air striping | 4425–4860 1) | 0.1 | AS | 95 | 20 | 2 | - | 70 2) | 94 3) | [9] |
| air striping | 5 | - | 60 2) | 50 3) | ||||||
| - | SBR | 0.16 | SBR | 8 | 20 ± 1 | 1 | >90 | 90 | >95 | [25] |
| 2 | >90 | 80–90 | >95 | |||||||
| 5 | >90 | 65–85 | 70–90 | |||||||
| 10 | >90 | 60–70 | 60–85 | |||||||
| influent | 4150 | 730.8 | - | - | - | - | - | - | - | [12] |
| +air striping | - | - | - | - | 25 ± 2 | - | 5.5 | 21.1 | 96.6 | |
| +Fenton | - | - | SBR | - | - | 15.3 | 60.8 | 97.4 | ||
| +SBR | - | - | - | 8 | 254) | 82.8 | 83.1 | 97.9 | ||
| coagulation | - | - | - | - | - | 84.5 | 93.3 | 98.3 | ||
SBR—Sequencing batch reactors, AS—Activated sludge system, SCFB—Semi-continuously fed batch, CF—Continuous-flow activated sludges with recycle. 1) before air pretreatment; 2) for soluble chemical oxygen demand (SCOD); 3) for the total ammoniacal nitrogen (TAN); 4) Effluent from the Fenton process was mix with municipal sewage wastewater

Table A4.
Characteristic of effluents (the highlighted text indicates the limit value given by Polish legislation; the values marked light grey and dark grey (with white font)) are for the control reactor and reactors fed with mixtures with pretreated leachate, respectively).
Table A4.
Characteristic of effluents (the highlighted text indicates the limit value given by Polish legislation; the values marked light grey and dark grey (with white font)) are for the control reactor and reactors fed with mixtures with pretreated leachate, respectively).
| Addition of Leachate (% v/v) | BOD5 (mg/L)/(% Removal) | COD (mg/L)/(% Removal) | TSS (mg/L)/(% Removal) | Total N (mg/L)/(%Removal) | Total P (mg/L)/(% Removal) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBR1 | SBR2 | SBR1 | SBR2 | SBR1 | SBR2 | SBR1 | SBR2 | SBR1 | SBR2 | |
| 0 | 2.55/99 | 2.55/99 | 71/80 | 71/80 | 0.89/99 | 0.89/99 | 5.4/82 | 5.4/82 | 0.47/93 | 0.47/93 |
| 5 | 5.3/98 | 5.3/95.5/98 | 96.01/77 | 83.5/80 | 5.9/95 | 1.18/99 | 20.2/71 | 13.9/80 | 0.8/88 | 0.8/89 |
| 10 | 22/92 | 5.5/98 | 165/72 | 129/78 | 14.7/90 | 4.4/97 | 34.968 | 22.9/79 | 1.5/79 | 1.1/87 |
| 15 | 31.4/89 | 14.25/95 | 227/70 | 166/78 | 33.5/81 | 8.8/95 | 59.4/60 | 37.1/75 | 1.9/75 | 1.5/83 |
| 20 | 59/80 | 17.7/94 | 383/65 | 274/75 | 61.7/70 | 22.6/89 | 90.2/52 | 56.4/70 | 2.8/65 | 1.8/80 |
| 25 | 82.3/73 | 24.4/92 | 449/65 | 333/74 | 84.6/64 | 50/80 | 111/51 | 72.8/68 | 3.1/63 | 2.3/75 |
| 30 | 110/65 | 34.7/89 | 585/60 | 395/73 | 106/60 | 58/78 | 147/45 | 101/62 | 3.3/62 | 2.5/75 |
| 35 | 137/58 | 78/76 | 791/52 | 495/70 | 117/60 | 76.2/74 | 169/45 | 111/62 | 3.46/62 | 2.7/74 |
| 40 | 194/42 | 101/70 | 1100/40 | 588/68 | 148/54 | 96.7/70 | 211/39 | 156/55 | 3.8/60 | 3/72 |
| 45 | 224/35 | 110/68 | 139,231 | 807/60 | 200/43 | 130/63 | 289/25 | 220/43 | 4/60 | 3.5/69 |
References
- Sewwandi, B.G.N.; Wijesekara, H.; Rajapaksha, A.U.; Mowjood, M.I.M.; Vithanage, M. Risk of soil and water pollution by heavy metals in landfill leachate. In Proceedings of the 12th Annual Conference of Thai Society of Agricultural Engineering, Chonburi, Thailand, 31 March–1 April 2011; Available online: https://www.researchgate.net/publication/265596878_Risk_of_Soil_and_Water_Pollution_by_Heavy_Metals_in_Landfill_Leachate (accessed on 9 January 2018).
- Grosser, A.; Jelonek, P.; Neczaj, E. Trends in the treatment of landfill leachate. In Interdisciplinary Problems in Engineering and Environmental Protection, 1st ed.; Wiśniewski, J., Kutyłowska, M., Trusz-Zdybek, A., Eds.; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Poland, 2015; Volume 5, pp. 95–124. Available online: https://www.eko-dok.pl/2015/10.pdf (accessed on 9 January 2018). (In Polish)
- Torretta, V.; Ferronato, N.; Katsoyiannis, I.A.; Tolkou, A.K.; Airoldi, M. Novel and conventional technologies for landfill leachates treatment: A review. Sustainability 2016, 9, 9. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.K.; Kumar, M. Suitability of Microwave and Microwave-coupled Systems for Landfill Leachate Treatment: An Overview. J. Environ. Chem. Eng. 2017, 5, 6165–6178. [Google Scholar] [CrossRef]
- Yuan, Q.; Jia, H.; Poveda, M. Study on the effect of landfill leachate on nutrient removal from municipal wastewater. J. Environ. Sci. 2016, 43, 153–158. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, F.A.; Kamel, G. Characterization and biological treatment of pre-treated landfill leachate. Ecol. Eng. 2016, 94, 268–274. [Google Scholar] [CrossRef]
- Amor, C.; De Torres-Socías, E.; Peres, J.A.; Maldonado, M.I.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, F.M.; Bruni, A.T.; Povinelli, J.; Vieira, E.M. Leachate/domestic wastewater aerobic co-treatment: A pilot-scale study using multivariate analysis. J. Environ. Manag. 2016, 166, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, S.; Gu, X.; Wang, K. Pilot study on the advanced treatment of landfill leachate using a combined coagulation, fenton oxidation and biological aerated filter process. Waste Manag. 2009, 29, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.; Fonseca, A.; Saraiva, I.; Vilar, V.J.; Boaventura, R.A. Biodegradability enhancement of a leachate after biological lagooning using a solar driven photo-Fenton reaction, and further combination with an activated sludge biological process, at pre-industrial scale. Water Res. 2013, 47, 3543–3557. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.S.; Abbas, A.A.; Chen, Y.P.; Liu, Z.P.; Fang, F.; Chen, P. Treatment of landfill leachate using a combined stripping, Fenton, SBR, and coagulation process. J. Hazard. Mater. 2010, 178, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Neczaj, E. Ultrasound Improvement of Landfill Leachate Biological Treatment Process, 1st ed.; Wydawnictwo Politechniki Czestochowskiej: Czestochowa, Poland, 2010; pp. 1–106. (In Polish) [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- USEPA—United States Environmental Protection Agency. Method 1683—Specific Oxygen Uptake Rate; EPA-821-R-01-014; USEPA: Washington, DC, USA, 2001. Available online: https://www.epa.gov/sites/production/files/2015-10/documents/method_1683_draft_2001.pdf (accessed on 9 January 2018).
- Yin, J.; Tan, X.J.; Ren, N.Q.; Cui, Y.B.; Tang, L. Evaluation of heavy metal inhibition of activated sludge by TTC and INT-electron transport system activity tests. Water Sci. Technol. 2005, 52, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Lo, S.L.; Kuo, J.; Yeh, C.J. Microwave-enhanced persulfate oxidation to treat mature landfill leachate. J. Hazard. Mater. 2015, 284, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Pagano, M.; Volpe, A.; Di Pinto, A.C. Fenton’s pre-treatment of mature landfill leachate. Chemosphere 2004, 54, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Cortez, S.; Teixeira, P.; Oliveira, R.; Mota, M. Mature landfill leachate treatment by denitrification and ozonation. Process Biochem. 2011, 46, 148–153. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Ban, Y.; Ren, B. A comparative study of UV–Fenton, UV–H2O2 and Fenton reaction treatment of landfill leachate. Environ. Technol. 2011, 32, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.B.; Clifford, E.; Devroedt, C.; Morrison, L.; Healy, M.G. Treatment of landfill leachate in municipal wastewater treatment plants and impacts on effluent ammonium concentrations. J. Environ. Manag. 2017, 188, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Order of the Minister of Environment of 18 November 2014 Laying Down Conditions for the Introduction of Sewage into Water Bodies or Soil and Laying Down the List of Substances Particularly Harmful to Water Environments. 2014. Available online: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU2 0140001800/O/D20141800.pdf (accessed on 9 January 2018). (In Polish)
- Ceçen, F.; Aktas, O. Effect of PAC addition in combined treatment of landfill leachate and domestic wastewater in semi-continuously fed batch and continuous-flow reactors. Water SA 2001, 27, 177–188. [Google Scholar] [CrossRef][Green Version]
- Çeçen, F.; Çakıroğlu, D. Impact of landfill leachate on the co-treatment of domestic wastewater. Biotechnol. Let. 2001, 23, 821–826. [Google Scholar] [CrossRef]
- Fudala-Ksiazek, S.; Luczkiewicz, A.; Fitobor, K.; Olanczuk-Neyman, K. Nitrogen removal via the nitrite pathway during wastewater co-treatment with ammonia-rich landfill leachates in a sequencing batch reactor. Environ. Sci. Pollut. Res. 2014, 21, 7307–7318. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).