1. Introduction
UV-based micropollutant decomposition methods like photolysis and heterogeneous photocatalysis belong to one of the most well-described water treatment processes in the literature. Their effectiveness depends on many factors related to the physicochemical parameters of the treated water solutions [
1] and the operational parameters of the decomposition process [
2]. It is very important to choose the source of UV radiation and to estimate the appropriate wavelength, the energy of which allows for the complete decomposition of different types of organic contaminants. The performance of UV-based processes, especially in water matrices with a high content of various organic compounds, can led to the formation of decomposition intermediates, which can strongly increase the toxicological risk of the post-processed water [
3]. Therefore, choosing the type of UV lamp becomes an important issue in water treatment processes.
The present research concerned estimation of the influence of UV irradiation spectra of four commercial UV lamps with a power of 150 W on the decomposition of various types of organic micropollutants.
2. Material and Methods
2.1. Water Samples
The subject of the study constituted water solutions (prepared based on deionized water with a conductivity of 18 MΩ cm−1) of chosen organic micropollutants with the concentration of 500 µg L−1. The analytical standards of all tested compounds, i.e., benzocaine (BE), carbamazepine (CBZ), acridine (ACR), butylated hydroxytoluene (BHT), caffeine (CAF), triallate (TRI), triclosan (TCS), oxadiazon (ODZ), β-estradiol (E2), 17α-ethinylestradiol (EE2), mestranol (EEME), and progesterone (P4) of purity grade >97.0%, were supplied by Sigma-Aldrich (Poznań, Poland). The pH of the prepared water solutions was adjusted to 7 using 0.1 mol L−1 NaOH. Experiments for all tested micropollutants were carried out separately.
2.2. Photodecomposition Processes
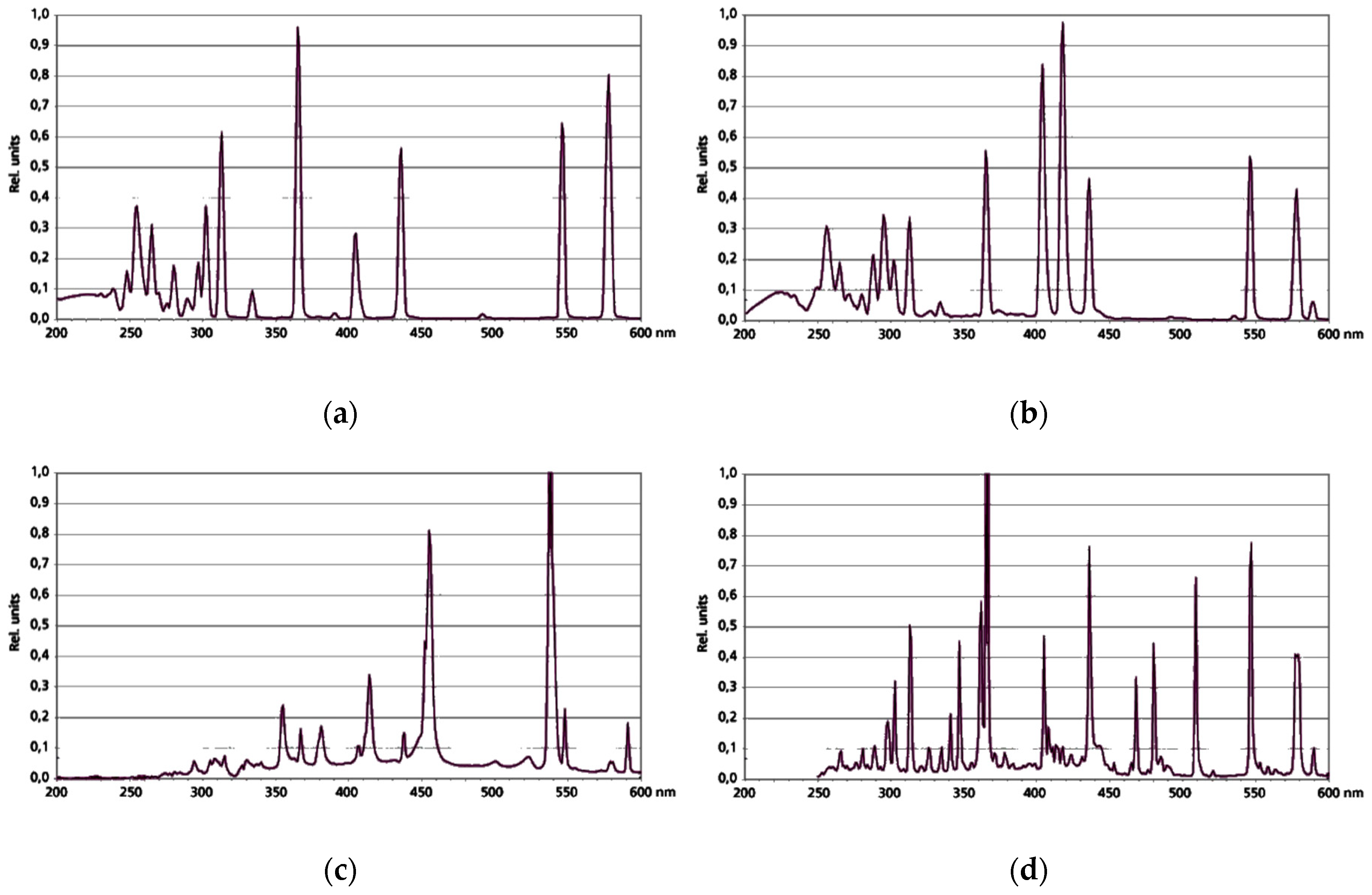
The photodecomposition processes were carried out in a laboratory glass batch reactor with a volume of 0.7 L from Heraeus (Hanau, Germany). The reactor was optionally equipped with four medium-pressure UV mercury vapor lamps (TQ, TQ-Z1, TQ-Z2, TQ-Z3) with a power of 150 W. The UV lamps were characterized by different wavelengths, λ
exc, of radiation given by the producer (
Figure 1). The processes of photolysis and heterogeneous photocatalysis were carried out for 5, 15, 30, and 60 min. The reaction mixture was aerated by an aeration pump with a capacity of 4 L of air per minute. Titanium dioxide from Evonik Degussa GmbH (Hanau, Germany) at the dose of 50 mg L
−1 was used as a process catalyst and separated from the reaction mixture after the irradiation process using a microfiltration set equipped with membrane filters by Merck Milipore (Darmstadt, Germany).
The analytical procedure of the tested compounds was performed using gas chromatography–mass spectrometry (GC-MS) with electron ionization preceded by solid-phase extraction (SPE). Details of the method are given in [
3]. Assignment errors were estimated on the basis of the standard deviation for three repetitions of each test. The kinetic of the process was calculated with the Langmuir–Hisherwood (L–H) equation [
4]. The tests were completed with a toxicological analysis performed using the Microtox
® test.
3. Results and Discussion
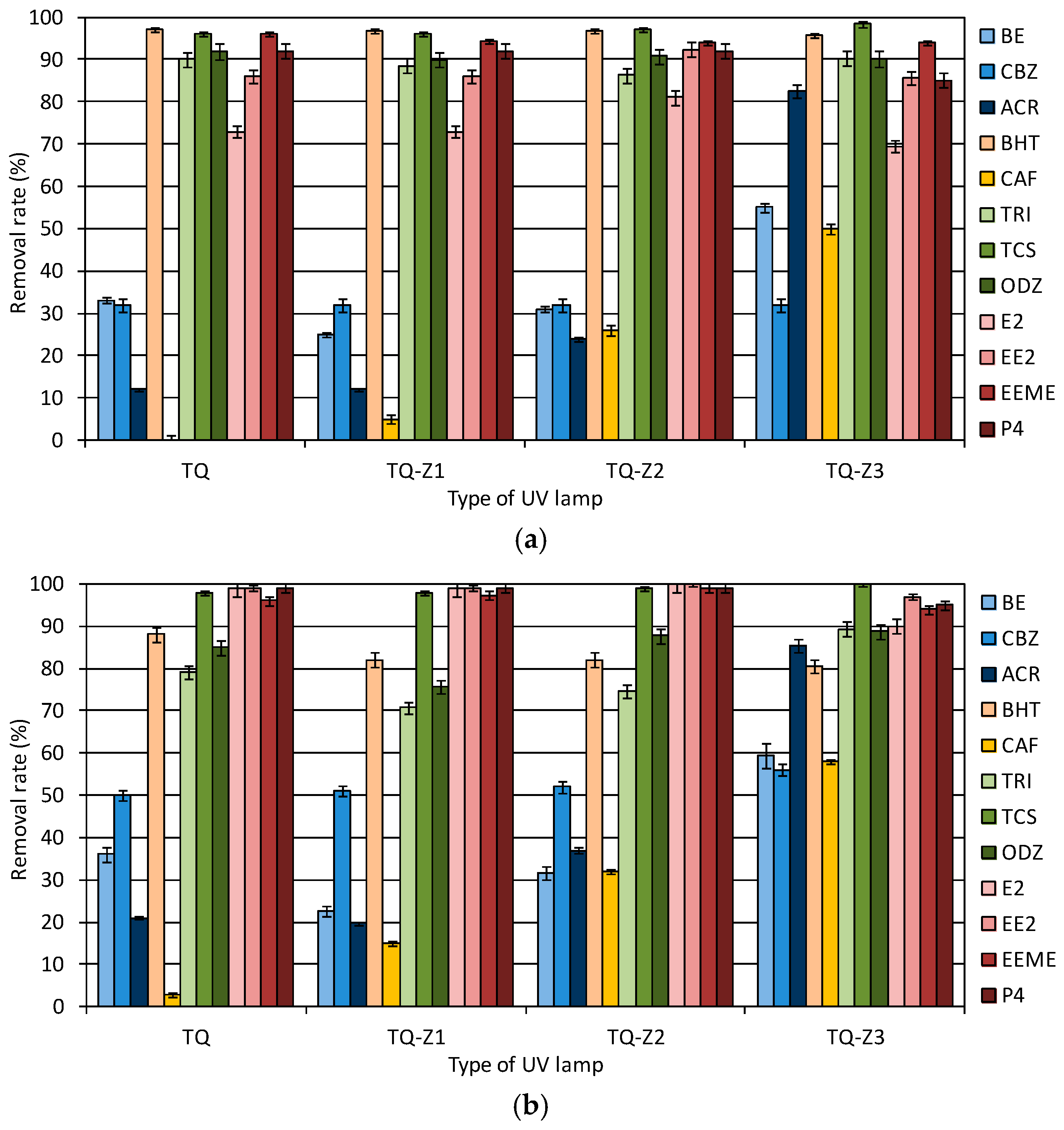
Figure 2 shows the reduction in the concentration of micropollutants tested after 60 min of exposure to different types of UV lamps. The tests were conducted with and without (photolysis) the presence of the TiO
2 catalyst.
The catalyst increased the decomposition of the tested micropollutants. Only in the case of TRI and ODZ were higher removal rates observed during the photolysis process. The type of UV lamp affected the rapidity of micropollutant decomposition. For example, the CAF concentration was reduced by more than 58% during the photocatalysis process carried out using the TQ-Z3 lamp, while the use of the TQ lamp caused only a 3% removal of this compound. The highest removal rates of compounds from the group of hormones (E2, EE2, EEME, P4) and pharmaceuticals (BE, CBZ, ACR) were noted for the TQ-Z3 lamp. Meanwhile, BHT was most effectively removed during exposure to UV light emitted by the HG lamp.
The chromatographic analysis of post-processed solutions indicated the formation of several decomposition intermediates, which were identified based on their mass spectrum using the NIST 17 database software. It was found that UV lamp irradiation spectra not only affected the ability and speed of compound decomposition but also the type of decomposition intermediates. The largest number of intermediates was produced in water solutions irradiated by the TQ lamp. For example, during the photocatalytic decomposition of CBZ using the TQ lamp, five by-products were formed: 3-hydroxycarbamazepine, 10,11-dihydro-10-hydroxycarbamazepine, dihydrocarbamazepine-10,11-trans-diol, 9-acridone, and acridine. In contrast, the process conducted with the TQ-Z2 or TQ-Z3 lamp only produced three intermediates: 3-hydroxycarbamazepine, dihydrocarbamazepine-10,11-trans-diol, and acridine.
Furthermore, toxicological analysis indicated the toxic nature of some of the decomposition by-products. Results of the toxicological assessment for water samples after 60 min of photolysis and photocatalysis are presented in
Table 1. Solutions were classified according to the toxicity classification of Mahugo-Santana et al. [
5].
4. Conclusions
On the basis of the studies conducted on the photodecomposition of different types of micropollutants, it can be concluded that the radiation wavelengths emitted by UV lamps of the same power determines the intensity of micropollutant decomposition. The presence of TiO2 photocatalyst increased the decomposition of organic compounds regardless of the type of UV lamp used. Only in the case of BHT, TRI, and ODZ was an inverse relationship observed. GC-MS analysis indicated the generation of several micropollutant decomposition by-products. The highest number of intermediates was produced in water solutions irradiated by the TQ lamp. Toxicological analysis of post-processed water samples revealed that some of the newly formed intermediates had a toxic potential. This led to an increase in the toxicity of water samples after the UV irradiation process.