1. Introduction
A range of solvents can be employed in the extraction process used in oil refining including (di/tri/tetra)ethylene glycol (D/T/TT/EG), diglycol amine (DGA), N-methyl pyrolidone (NMP), dimethylsulphoxide (DMSO), dimethylformamide (DMF), morpholine, and carbonate derivatives. An attractive alternative to commonly used industrial extractive liquids is sulfolane. Considering the physicochemical properties, sulfolane is an attractive aprotic solvent that is widespread in the industry.
The main purpose of the pilot-case study was to verify the applicability of the industrial, multi-electrochemical technology for reliable detection of corrosion processes in the fluids with low conductivity. Several aspects of the corrosion measurement were taken into account, including the influence of process parameters (temperature as well as the impact of impurities, e.g., water, oxygen, and chlorides) on the corrosion of carbon steel in pure sulfolane. In our studies we used the SmartCET
® by Honeywell that is based on low frequency impedance (LFI), harmonic distortion analysis (HDA), and electrochemical noise (ECN). The industrial, wired transmitter model CET5500 was used conjugated with the HART (Highway Addressable Remote Transducer) system. The set of the flat coupon electrodes made of AISI 1010 carbon steel was used (dimensions: 89 × 20 × 2 mm) in the study. Hence, a dedicated testing vessel was designed and constructed. The electrodes were supported on a special grooved glass frame to ensure their stability during the experiment and to maintain contact with the solvent. Several aspects of the corrosion measurement of general and localized corrosion modes were scrutinized extensively [
1]. The incipient attempts to quantify impact of process parameters (temperature) and impurities (e.g., water, oxygen, and chlorides) on carbon steel corrosion in the pure sulfolane provided meaningful data, where at tested temperature ranging from 95 to 240 °C, carbon steel showed relatively low corrosion rates. Moreover, the oxygen ingress to the pure sulfolane showed minimal impact on the corrosion rates but engendered enhanced localized corrosion activity. As we observed, chlorides enhanced both general and localized corrosion, respectively. The obtained findings for carbon steel demonstrate the applicability of multi-technique electrochemical monitoring systems for rapid and accurate detection of sulfolane corrosion [
2].
Sulfolane (C
4H
8SO
2) is a five-membered, heterocyclic sulfur-organic compound that is easily soluble in water (see
Figure 1). Sulfolane is the generic name for hydrogenated sulfones of butadiene also known under a variety of synonyms/numbers including thiolane 1,1-dioxane (IUPAC), 2,3,4,5-tetrahydrothiophene-1,1-dioxide (systematic), thiocyclopentane-1,1-dioxide, (cyclo) tetramethylene sulphone, dihydrobutadiene sulphone, sulphoxaline, 126-33-0 (CAS), or 204-783-1 (EINECS), respectively.
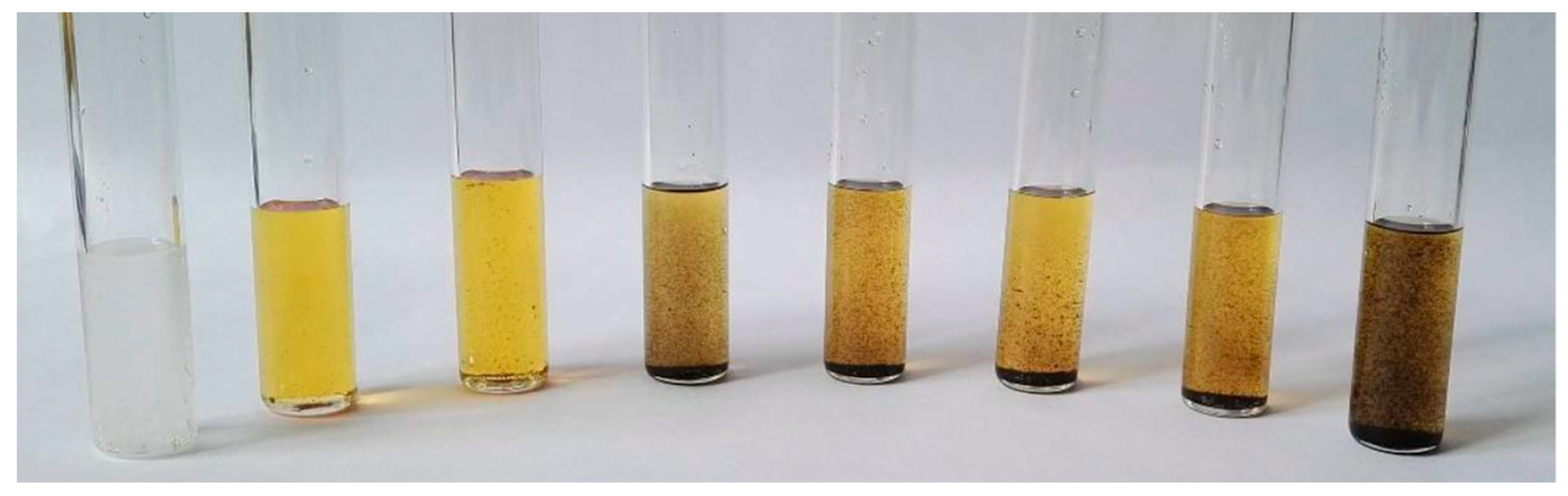
In industry, sulfolane can be colored from a bright yellow to dark yellow according to water and air content as illustrated in
Figure 2 [
3].
Sulfolane does not volatilize in water or soil, nor it is not subject to rapid degradation by organic matter. Research indicates that sulfolane is present in the industrial waste water from refineries and natural gas processing plants [
3]. It is still questionable whether sulfolane is corrosive towards industrial pipelines since corrosion of tanks or vessels is usually found during routine turnarounds.
Pure sulfolane is not considered to be a substance causing or accelerating corrosion or the destruction of steel. The corrosion of steel associated with the use of sulfolane is the result of the by-products produced by the decomposition of sulfolane. Some general correlations between oxygen and chloride concentration leading to sulfolane corrosivity are known. Sulfolane is thermally stable up to about 220 °C when it begins to break down into sulfur dioxide and a polymeric material.
2. Materials and Methods
The main purpose of the research conducted on carbon steel AISI 1010 was preliminary analysis of selected factors that could potentially affect the low-conductivity corrosion rate. Monitoring of specific parameters allows the determination of trends, similarities, and differences between general and local corrosion mechanisms. Laboratory tests of the impact of process parameters (temperature) and pollutants (oxygen and chloride) on the corrosion of carbon steel in pure sulfolane provided important data and clues that may support the practical application in large-scale industrial processes [
2]. All measurement results were recorded using the industrial electrochemical monitoring system SmartCET, which uses low frequency impedance (LFI), harmonic distortion analysis (HDA), and electrochemical noise (ECN). Each parameter, e.g., general corrosion rate, local corrosion potential (pitting factor), current Stern–Geary coefficient—B value, and corrosion mechanism index (CMI) were measured and recorded at 60 second intervals using the appropriate data recording system. The experiment time was set to 96 hours on the non-standard electrodes made of carbon steel AISI 1010. A series of corrosion tests were carried out, maintaining the process temperature in the range of 95 °C, 180–190 °C, and 230–240 °C with the pure sulfolane. During the experiments with the addition of oxygen, air was introduced under the surface of the sulfolane solution in the appropriate quantities within 3 days. The influence of chlorides (50 ppm) on the steel corrosion was examined as well.
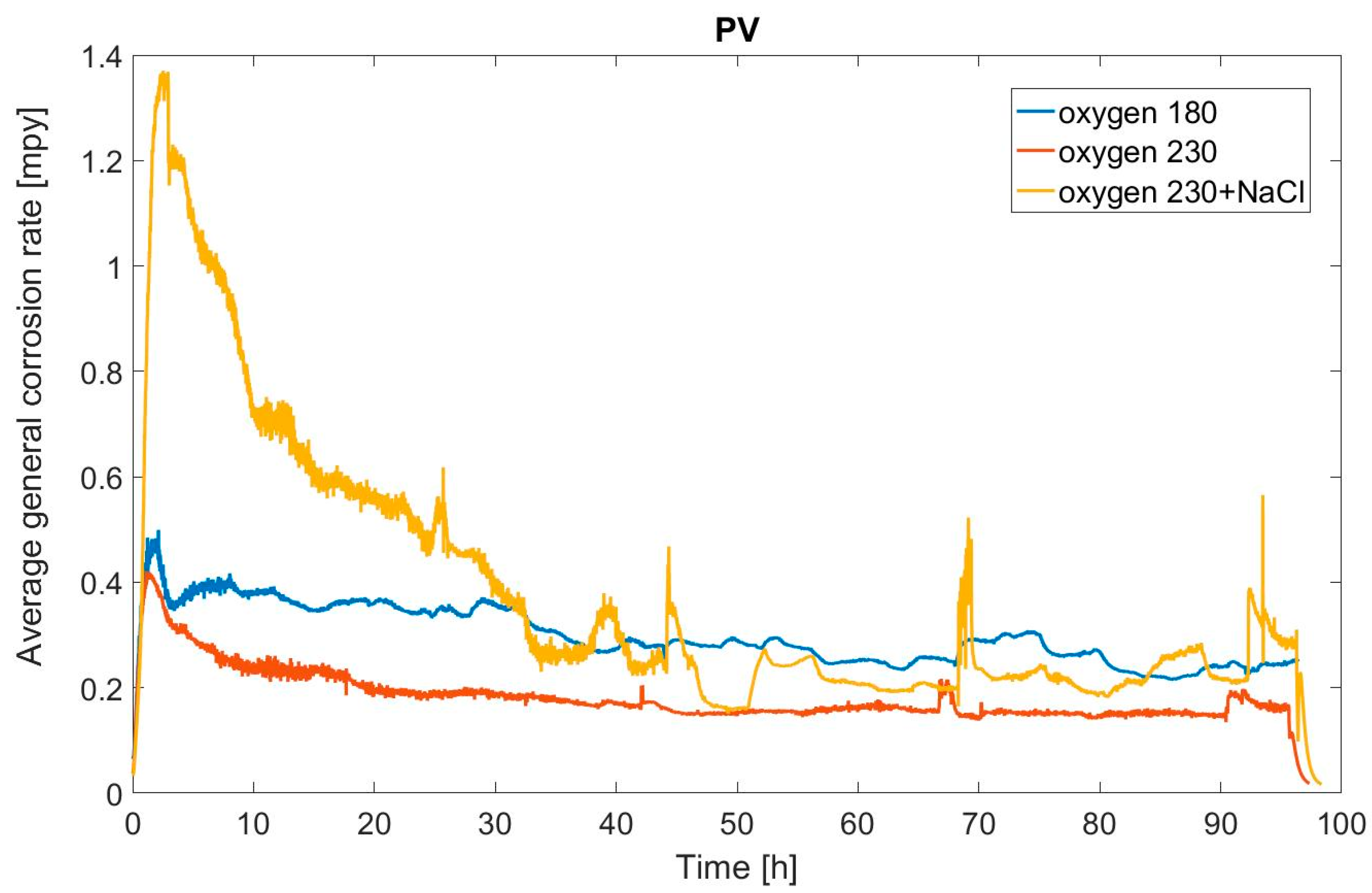
General corrosion rate increases with temperature, but even at 230 °C the maximum corrosion rate does not exceed 1 mpy as illustrated in
Figure 3. Basically, the ingress of oxygen does not increase noticeably the sulfolane general corrosion rate; however, the addition of chlorides clearly increased the corrosion rate when compared to water and water/oxygen conditions.