Experimental Research on the Air Gasification of Oily Sawdust †
Abstract
:1. Introduction
1.1. Research Purpose
1.2. Gasification Process
2. Materials and Methods
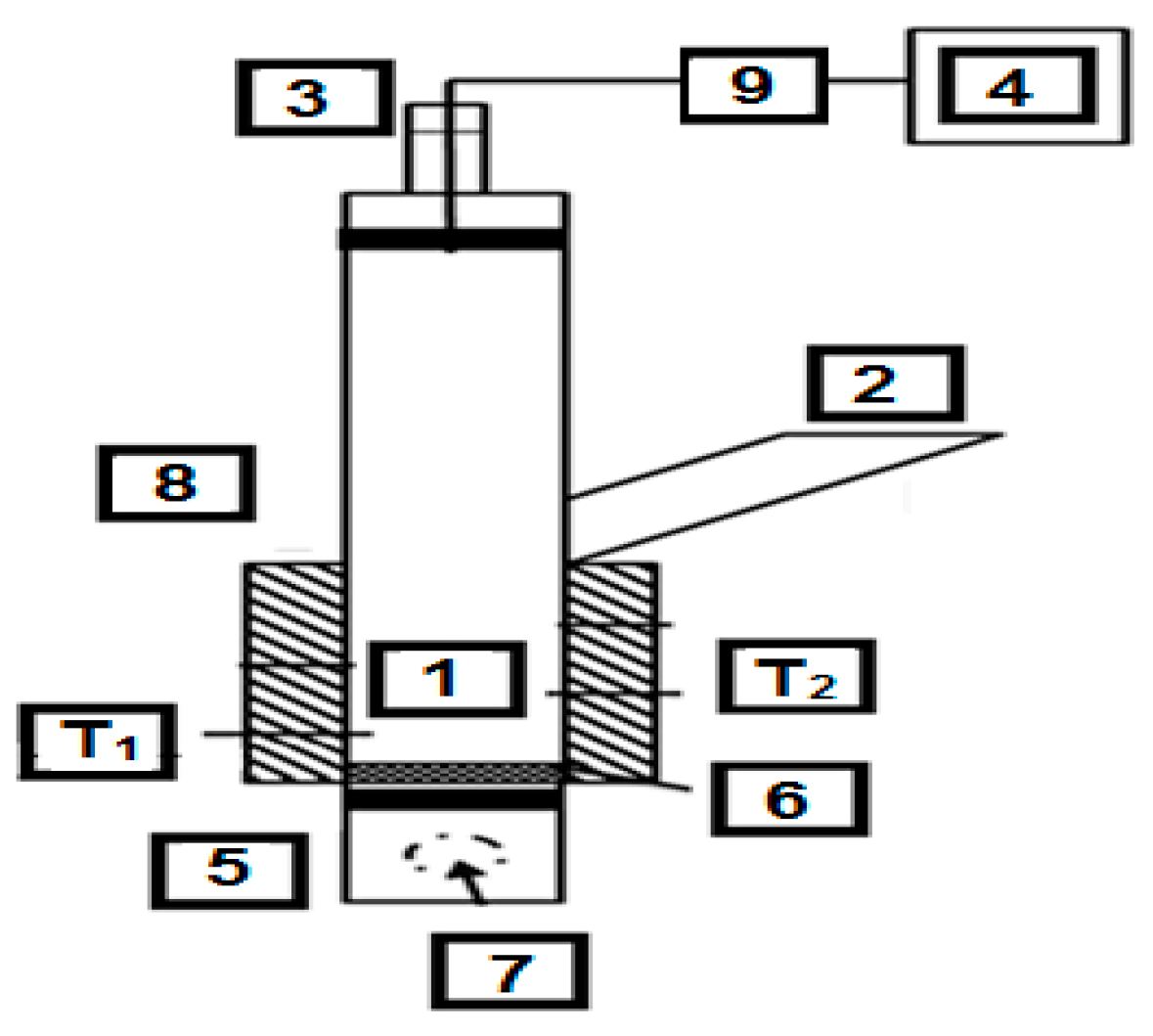
2.1. Research Placement
2.2. Methanization Coefficients Evaluation
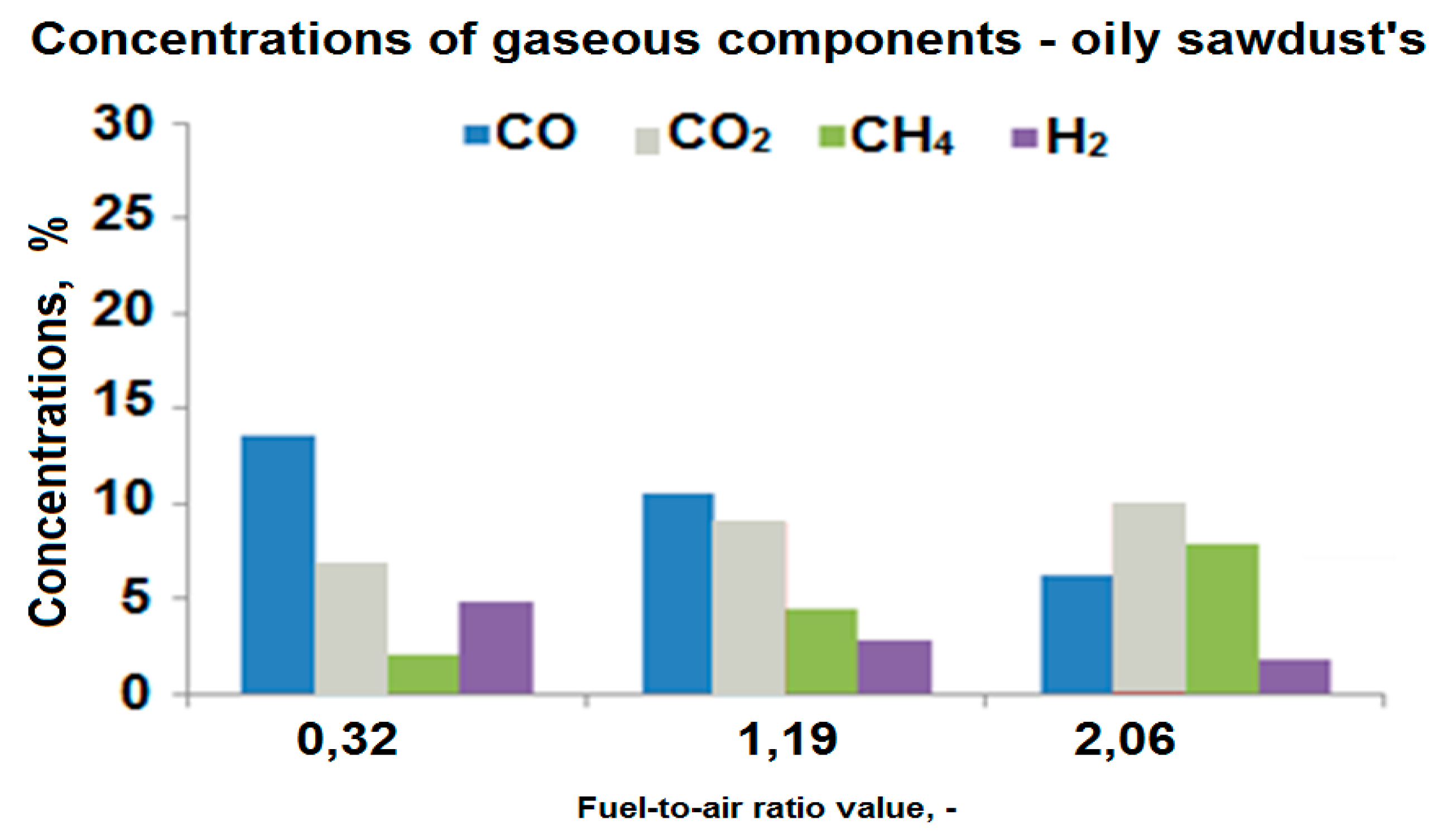
3. Results
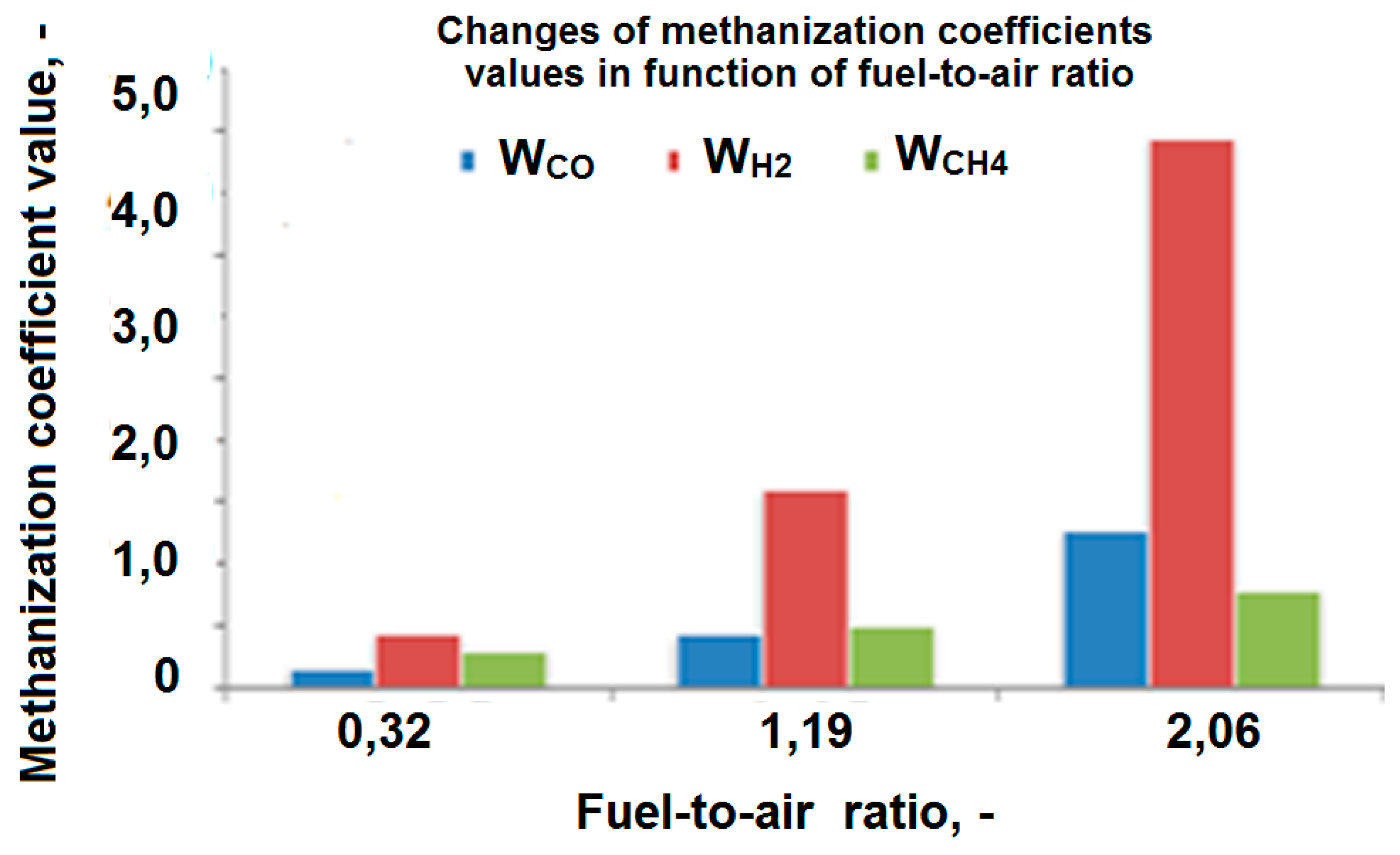
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Waste Act of 14th December 2012 (in Polish) Ustawa z dnia 14 Grudnia 2012 o odpadach (Dz.U.2013poz.21). Available online: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU20130000021/T/D20130021L.pdf (accessed on 28 June 2019).
- Hawrot-Paw, M.; Koniuszy, A.; Mikicuk, M.; Izwikow, M.; Stawicki, T.; Sędłak, P. Analysis of ecotoxic influence of waste from the biomass gasification process. Environ. Sci. Pollut. Res. 2017, 24, 15022–15030. [Google Scholar] [CrossRef] [PubMed]
- Directive of the European Parliament and of the European Council 2008/98/EC of November 19, 2008 on Waste Repealing Certain Directives (Dz.U.L.312 dated November 22, 2008). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0098 (accessed on 28 June 2019).
- Bach-Oller, A.; Furusjo, E.; Umeki, K. Fuel conversion characteristics of black liquor and pyrolysis oil mixtures: Efficient gasification with inherent catalyst. Biomass Bioenergy 2017, 79, 155–165. [Google Scholar] [CrossRef]
- Sharma, T.; Yepes Maya, D.M.; Nascimento, F.R.M.; Yunye, S.; Ratner, R.; Silva Lora, E.E.; Mendes Neto, L.J.; Escobar Palacios, J.C.; Andrade, R.V. An Experimental and Theoretical Study of Miscanthus Briquettes in a Double-Stage Downdraft Gasifier: Syngas, Tar and Biochar Characterization. Energies 2018, 11, 3225–3324. [Google Scholar] [CrossRef]
- Grzywa, E.; Molenda, J. Technology of Basic Organic Synthesis; Wydawnictwo Naukowo-Techniczne: Warszawa, Poland, 1987. (In Polish) [Google Scholar]
- Emami-Taba, L.; Faisal Irfan, M. Fuel blending effects on the co-gasification of coal and biomass—A review. Biomass Bioenergy 2013, 57, 249–263. [Google Scholar] [CrossRef]
- Frusteri, F.; Frusteri, L.; Costa, F.; Mezzapica, A.; Cannilla, C.; Bounara, G. Methane production by sequential supercritical gasification of aqueous organic compounds and selective CO2 methanation. Appl. Catal. A Gen. 2017, 545, 24–32. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| C(dry basis), % | 56.31 |
| H(dry basis), % | 6.75 |
| N(dry basis), % | 0.85 |
| S(dry basis), % | 0.13 |
| Cl(dry basis), % | 0.33 |
| O(dry basis), % | 20.61 |
| Combustible substance, % | 84.98 |
| Moisture (as given), % | 6.00 |
| LHV(dry basis), MJ/kg | 23.626 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałko, G.; Król, D. Experimental Research on the Air Gasification of Oily Sawdust. Proceedings 2019, 16, 33. https://doi.org/10.3390/proceedings2019016033
Gałko G, Król D. Experimental Research on the Air Gasification of Oily Sawdust. Proceedings. 2019; 16(1):33. https://doi.org/10.3390/proceedings2019016033
Chicago/Turabian StyleGałko, Grzegorz, and Danuta Król. 2019. "Experimental Research on the Air Gasification of Oily Sawdust" Proceedings 16, no. 1: 33. https://doi.org/10.3390/proceedings2019016033
APA StyleGałko, G., & Król, D. (2019). Experimental Research on the Air Gasification of Oily Sawdust. Proceedings, 16(1), 33. https://doi.org/10.3390/proceedings2019016033






