1. Introduction
Stabilization and polymer flocculation are effects of the adsorption of macromolecular compounds on the surface of particles in the dispersed phase or the presence of unadsorbed polymer macromolecules in the dispersing phase. The adsorbed polymer can form loops and tails, so that only a few fragments of the polymer chains come into direct contact with the solid surface. The addition to a system of a certain amount of polymer that will not provide complete coverage of the surface contributes to the formation of polymer bridges. This is only possible if the range of electrostatic interaction between the particles is smaller than the length of the loops and tails of the polymer. As a result of bridging, the flocculation process takes place. Flocculation can also be the result of the neutralization of solid charge by polyelectrolyte molecules endowed with an opposite charge [
1,
2]. The phenomena of stabilization and flocculation through the addition of polymer is widely used in many branches of industry, e.g., agriculture. Polymer strengthens the cohesion of the soil by binding the loose mineral particles and flocculates the suspensions, thus limiting the process of soil erosion. Macromolecular compounds interact with the soil clay minerals and change their surface properties, which affects the fate of other compounds present in the surrounding environment. Therefore, the presence of flocculants may influence the occurrence of toxic compound accumulation, such as lead ion accumulation, which can cause various diseases and disorders in humans as well as environmental damage at very low concentrations [
3,
4]. The aim of the study was to investigate the influence of the presence of a cationic flocculant on lead(II) ion adsorption onto the surface of montmorillonite. The effect of the cationic group content in the polymeric macromolecules, the addition order of individual adsorbates, and the lead(II) ion concentration were also determined. Using spectrophotometry, potentiometric titration, and microelectrophoresis methods, the adsorption and electrokinetic properties of studied montmorillonite–CT PAM–Pb(II) systems were established.
2. Materials and Methods
Montmorillonite (2:1 clay mineral; Sigma-Aldrich, Saint Louis, MO, USA) with specific surface area of 259.1 m
2/g and a pore size equal to 5.9 nm was used as an adsorbent. A cationic polyacrylamide (Korona) with different contents of dissociated groups (35% and 80%) with molecular weights equal to 7000 kDa was also used. All adsorption measurements were carried out at pH = 5 ± 0.1 and 25 °C in the presence of 0.001 M NaCl. Adsorbed amounts of cationic polyacrylamide and lead(II) on the montmorillonite surface were determined by the static method based on the difference between the PAM and Pb(II) concentrations in the solutions before and after the adsorption process. In case of the cationic polyacrylamide, the polymer concentration was determined spectrophotometrically using a UV-VIS spectrophotometer (Carry 100; Varian) and brilliant yellow as an indicator at 495 nm. In order to determine the concentration of Pb, the reaction of Pb(II) ions with 4-(2-pyridylazo)-rezorcinol was used. The lead ion content was determined spectrophotometrically at λ = 520 nm. The surface charge density of montmorillonite without/with polymer and/or Pb(II) was determined using the potentiometric titration method [
5]. The changes in the soil suspension stability in the absence and presence polymer was monitored using spectrophotometry (Carry 100 spectrophotometer, Varian). The absorbance of the montmorillonite suspension in the absence and presence of cationic polyacrylamide was measured as a function of time at the wavelength λ = 500 nm.
3. Results and Discussion
The cationic polyacrylamide and Pb(II) ion adsorption amounts on the surface of montmorillonite are presented in
Table 1.
Higher adsorption was observed in case of CT PAM 80% in comparison to CT PAM 35%. The pKb values determined for the polyacrylamide samples were within 9.3–9.5 and at such a pH range that the degree of cationic group dissociation was 50%. At the studied pH value, the total charge of the solid surface is 0 and the degree of quaternary amine group dissociation was equal to 99.99%. For this reason, the higher adsorption affinity of CT PAM 80% was caused by favorable electrostatic attractions occurring between the positively charged adsorbing macromolecules and negatively charged solid surface. Consequently, the adsorption amount of polymer increased. Moreover, the adsorption of lead(II) ions was caused by two mechanisms: electrostatic attraction between solid hydroxyl groups and metal cations as well as the ion exchange process. The Pb(II) ions underwent ion exchange with interlayered cations present in the mineral structure. In the examined systems containing PAM with 35% or 80% cationic groups, both the order of adsorbates addition and the lead(II) ion concentration had a minimal effect on the amounts of CT PAM 35% and CT PAM 80% adsorbed on the montmorillonite surface. Such behavior was observed for all examined concentrations of Pb(II). At pH 5, both PAM samples exhibited a strongly developed conformation due to the presence of positively charged groups in the polymer chains. However, the cationic polymer macromolecules were too large in comparison to the pore size of the montmorillonite and thus they could not fully penetrate the internal mineral structure. Comparing the size of lead(II) ions and polymeric macromolecules of PAM, these cations had a small size and were able to freely penetrate the layered montmorillonite structure. Moreover, due to the electrostatic repulsion between the cationic polymer quaternary amine groups and the lead cations, a slight decrease in the adsorption amount of CT polymer in the presence of Pb(II) ions was observed.
The order of polymer and heavy metal ion addition to the studied system had a minimal effect on the Pb(II) ion adsorption on the montmorillonite surface. In the case of a 1 ppm concentration of lead(II) ions, the addition of the both cationic polyacrylamides did not affect the amount of lead cations adsorbed (
Table 2).
However, in the systems with lead(II) ions with concentrations of 10 or 100 ppm, a slight increase in their adsorption in the presence of CT PAM was observed. The free electron pairs located on the nitrogen atoms of PAM amide groups were probably involved in this adsorption increase due to the creation of covalent bonds between the amide groups and lead(II) ions [
6,
7]. This indicates that despite the electrostatic repulsion between the positively charged ionized groups of polymer and the Pb(II) cations, these heavy metals can be captured from the solution and the accumulation phenomenon can occur.
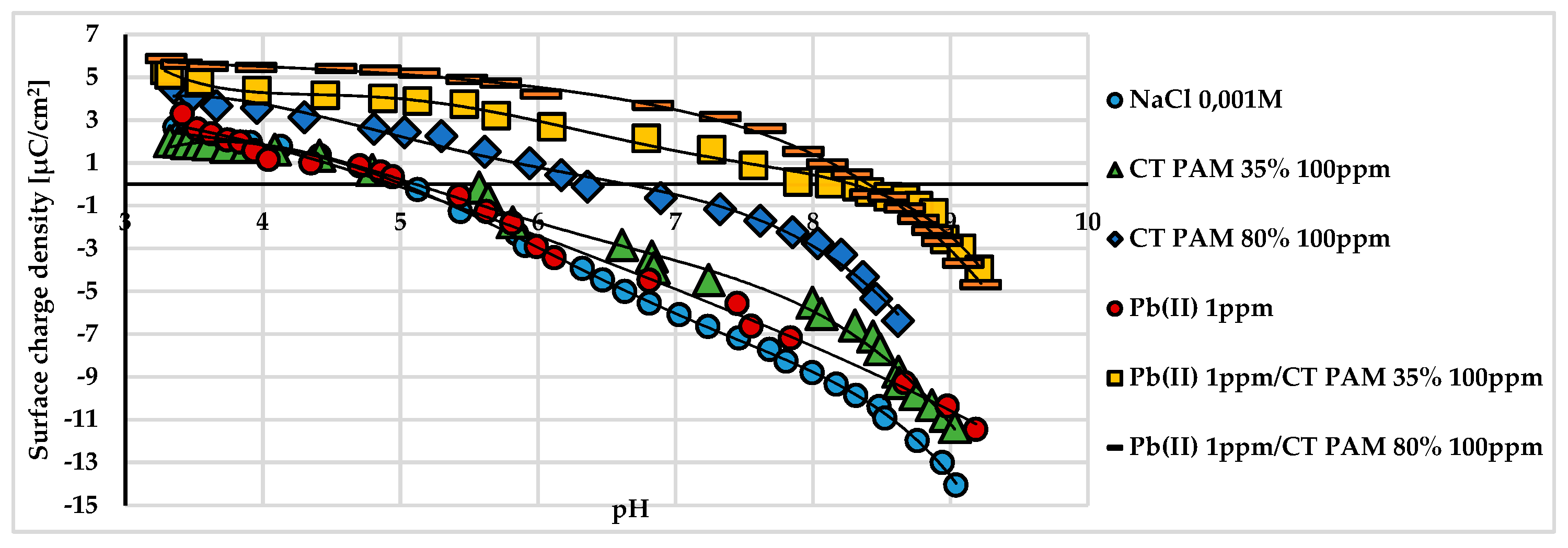
The presence of lead(II) cations in the system changed the surface charge density throughout the entire examined pH range (
Figure 1).
This was caused by the positively charged quaternary amine groups of PAM adsorbed on the solid surface. However, most of these groups were located in the by-surface layer of the solution in the tail and loop structures of the adsorbed polymer. Insignificant changes in the surface charge density of the polymer- and PAM/lead(II) ion-containing system were observed. It can be stated that the polymer–heavy metal complexes present in the interfacial layer assumed a spatial arrangement such that the lead(II) ions present in their structure cause only slight changes to the surface charge of the examined clay mineral.
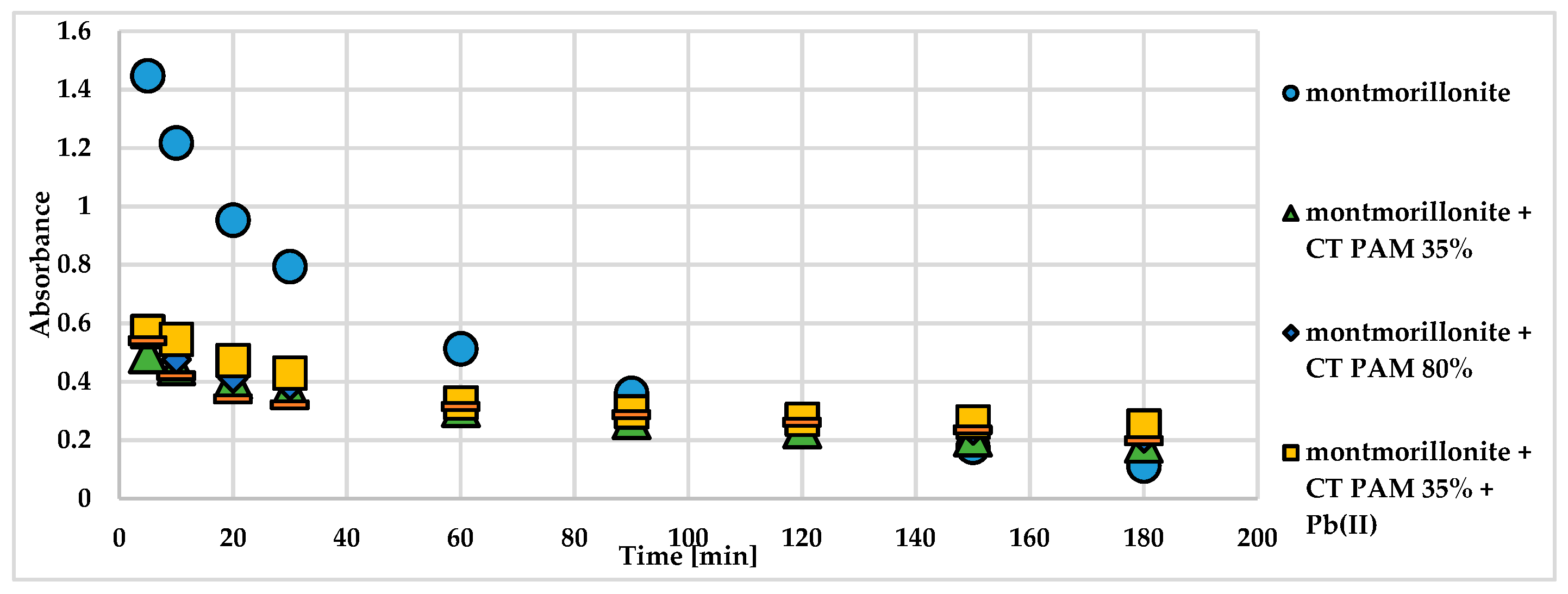
The addition of cationic polyacrylamide to the montmorillonite suspension caused considerable changes in the montmorillonite suspension stability at pH 5 (
Figure 2).
After only 5 minutes following the cationic polymer addition, the obtained absorbance values were small in comparison to those of the system without PAM. This is related to the formation of large sedimentation aggregates on the bottom of the vial. Thus, the cationic PAM has flocculating properties on the montmorillonite particles and can be used as a potential soil conditioner. Small changes in the stability properties of suspensions containing CT PAM 35% or 80% and Pb(II) ions in comparison to the systems without heavy metal ions were observed.
4. Conclusions
Based on the obtained results of adsorption, electrokinetic, and stability measurements, the following conclusions can be drawn:
- (1)
A higher adsorbed amount of cationic polyacrylamide on the montmorillonite surface was observed in the case of a polymer with a greater number of cationic groups due to more beneficial electrostatic forces.
- (2)
The addition order of the adsorbates had practically no effect on the adsorption quantities of both cationic polyacrylamide and lead(II) ions.
- (3)
The lead(II) ion concentration had a minimal effect on the amount of CT PAM 35% and CT PAM 80% adsorbed on the montmorillonite surface.
- (4)
Despite the electrostatic repulsion between the positively charged ionized groups of CT PAM and Pb(II) cations, these heavy metal can be captured from the solution due to creation of covalent bonds between the amide PAM groups and Pb(II) ions.
- (5)
The presence of lead(II) ions and cationic polyacrylamide affects the electrokinetic properties of montmorillonite.
- (6)
CT PAM has flocculating properties on the montmorillonite particles and can be used as a potential soil conditioner; however, it can cumulate heavy metal ions, which is a very undesirable phenomenon for the natural environment.