Influence of Ionic Liquids Assisted Synthesis on Morphology and Photocatalytic Properties of Bi4O5Br2 †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Bi4O5Br2
2.2. Photocatalytic Tests
3. Results
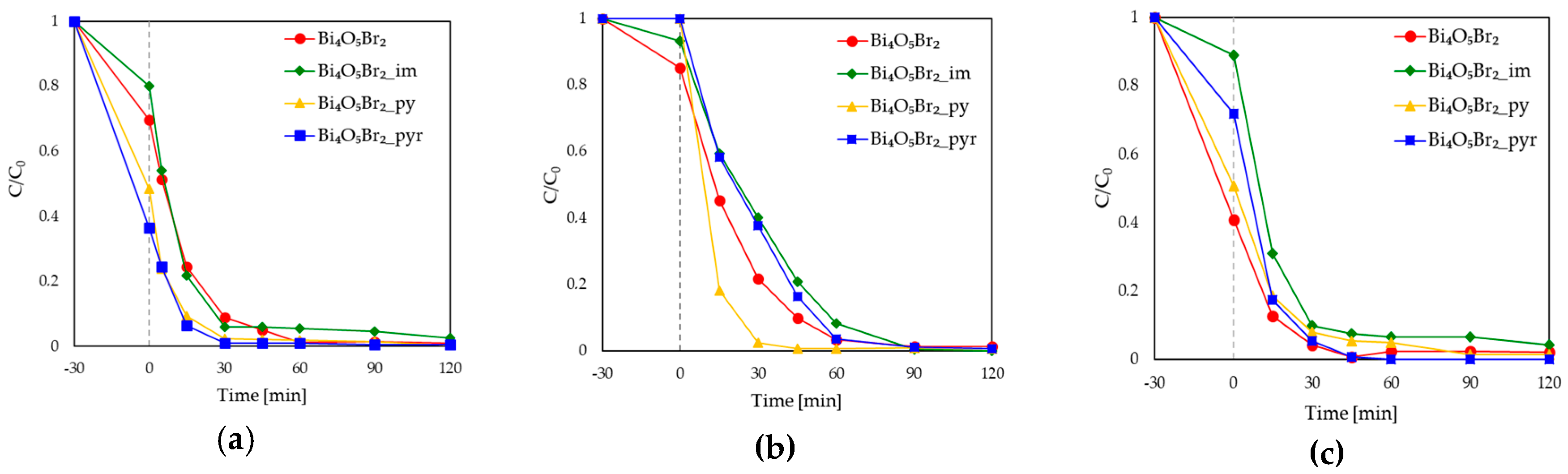
3.1. Photocatalytic Activity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- He, Z.; Paschalis, A. Ionic liquid and nanoparticle hybrid systems: Emerging applications. Adv. Colloid Interface Sci. 2017, 244, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Su, Y.; Jin, X.; Xie, H.; Zhang, C. Recent advances in BiOX (X = Cl, Br and I) photocatalysts: synthesis, modification, facet effects and mechanisms. Environ. Sci. Nano 2014, 1, 90–112. [Google Scholar] [CrossRef]
- Duan, X.C.; Ma, J.M.; Lian, J.B.; Zheng, W.J. The art of using ionic liquids in the synthesis of inorganic nanomaterials. CrystEngComm 2014, 16, 2550–2259. [Google Scholar] [CrossRef]
- Bielicka-Giełdoń, A.; Wilczewska, P.; Malankowska, A.; Szczodrowski, K.; Ryl, J.; Zielińska-Jurek, A.; Siedlecka, E.M. Morphology, Surface properties and photocatalytic activity of the bismuth oxyhalides semiconductors prepared by ionic liquid assisted solvothermal methods. Sep. Purif. Technol. 2019, in press. [Google Scholar] [CrossRef]
- Ye, L.Q.; Jin, X.; Liu, C.; Ding, C.; Xie, H.; Chu, K.H.; Wong, P.K. Thickness-ultrathin and bismuth-rich strategies for BiOBr to enhance photoreduction of CO2 into solar fuels. Appl. Catal. B 2016, 187, 281–290. [Google Scholar] [CrossRef]
- Jiang, G.H.; Wei, Z.; Chen, H.; Du, X.X.; Li, L.; Liu, Y.K.; Huang, Q.; Chen, W.X. Preparation of novel carbon nanofibers with BiOBr and AgBr decoration for the photocatalytic degradation of rhodamine B. RSC Adv. 2015, 5, 30433–30437. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczewska, P.; Bielicka-Giełdoń, A.; Borzyszkowska, A.F.; Pieczyńska, A.; Siedlecka, E.M. Influence of Ionic Liquids Assisted Synthesis on Morphology and Photocatalytic Properties of Bi4O5Br2. Proceedings 2019, 16, 26. https://doi.org/10.3390/proceedings2019016026
Wilczewska P, Bielicka-Giełdoń A, Borzyszkowska AF, Pieczyńska A, Siedlecka EM. Influence of Ionic Liquids Assisted Synthesis on Morphology and Photocatalytic Properties of Bi4O5Br2. Proceedings. 2019; 16(1):26. https://doi.org/10.3390/proceedings2019016026
Chicago/Turabian StyleWilczewska, Patrycja, Aleksandra Bielicka-Giełdoń, Agnieszka Fiszka Borzyszkowska, Aleksandra Pieczyńska, and Ewa Maria Siedlecka. 2019. "Influence of Ionic Liquids Assisted Synthesis on Morphology and Photocatalytic Properties of Bi4O5Br2" Proceedings 16, no. 1: 26. https://doi.org/10.3390/proceedings2019016026
APA StyleWilczewska, P., Bielicka-Giełdoń, A., Borzyszkowska, A. F., Pieczyńska, A., & Siedlecka, E. M. (2019). Influence of Ionic Liquids Assisted Synthesis on Morphology and Photocatalytic Properties of Bi4O5Br2. Proceedings, 16(1), 26. https://doi.org/10.3390/proceedings2019016026






