1. Introduction
The incidence of cancer continually affects the general population in both developed and developing areas. Early detection and screening allow appropriate treatment of the patient, depending on the stage of the disease. There are currently screening methods for cancers such as breast, colorectal, and cervical cancer. Breast cancer is the second most common worldwide, yet its mortality rate has been declining, which is due in part to effective screening methods such as mammography. However, in some cases this technique does not allow an efficient detection [
1,
2]. Biosensors are available tools for a more specific detection of some biomarkers. The development of biosensors and bioassays for the detection of breast cancer biomarkers in serum can contribute to this success. In serum analysis, biomarkers that shed extracellular domains (ECD) in the peripheral blood (e.g., Human Epidermal growth factor Receptor 2 (HER2)) are important analytes. Electrochemical strategies based on the use of nano- or micromaterials can improve the assays’ performance. The use of small size transducers, the reasonable short assay times and the low sample volumes are key features for the development of point-of-care (POC) devices.
Distinct electrochemical immunosensing strategies were successfully developed for the analysis of the extracellular domain of HER2 (HER2-ECD). A sandwich assay was performed using modified SPCEs. The addition of silver nitrate to the enzymatic substrate leads to the deposition of metallic silver which was stripped into solution by linear sweep voltammetry providing the analytical signal.
2. Materials and Methods
2.1. Instrumentation and Reagents
The electrochemical measurements were carried out using a potentiostat/galvanostat (PGSTAT101, Metrohm Autolab, Utrecht, The Netherlands) controlled by the NOVA software package (v.1.9; Metrohm Autolab, Utrecht, The Netherlands). Screen-printed carbon electrodes (SPCE, DRP-110) and a specific connector (DRP-CAC) to interface the electrodes and the potentiostat/ galvanostat were supplied by Metrohm DropSens (Oviedo, Spain). Monoclonal anti-human-HER2-ECD antibody (capture antibody), monoclonal biotinylated anti-human-HER2-ECD antibody (detection antibody), and a recombinant HER2-ECD protein were obtained from Sino Biological Inc. (Beijing, China) Dynabeads™ MyOne™ carboxylic acid (MB-COOH, 10 mg/mL) were acquired from Life Technologies (Carlsbad, United States of America). Reduced graphene oxide (rGO), carboxylic acid-functionalized multiwalled carbon nanotubes (MWCNT-COOH), carboxylic acid-functionalized single-walled carbon nanotubes (SWCNT-COOH), streptavidin-alkaline phosphatase (S-AP) from Streptomyces avidinii, human serum (from male AB clotted whole blood), were obtained from Sigma-Aldrich (Darmstadt, Germany). Working solutions of the capture (Ab-C) and detection (Ab-D) antibodies and the antigen were prepared in 0.1 M Tris-HNO3 pH 7.4. The EDC/NHS mixed solution was prepared in 0.1 M MES pH 5. The ethanolamine (1 M) blocking solution was prepared in PBS pH 8. The S-AP solutions were prepared in 0.1 M Tris-HNO3 pH 7.4 containing 1% BSA. The solution containing 3-IP (1.0 × 10−3 M) and silver nitrate (4.0 × 10−4 M) was prepared in 0.1 M Tris-HNO3 pH 9.8 containing Mg(NO3)2 (2.0 × 10−2 M).
2.2. Immunossay Development and Procedure
SPCE-based immunosensors were tested using different nanomaterials: rGO, SWCNT-COOH, MWCNT-COOH and AuNPs and combinations of these materials. For the SPCE surface modification, a 1-µg/µL suspension of each carbon nanomaterial was placed on the working electrode (WE) of the SPCE and allowed to dry at 50 °C. The modification with gold nanoparticles was carried out by electrodeposition of gold from a 0.1 mM [AuCl4]− (in HCl) solution through the application of a constant current, performed as described elsewhere [
3]. For the immunomagnetic assay, 30 µg of MBs-COOH was used, and an EDC/NHS solution was added (20 min). The capture antibody was immobilized on the modified SPCE surfaces or on the MBs surface, performed through: incubation with 25 µg/mL of the Ab-C solution, overnight at 4 °C. A washing step was performed before blocking the free surface sites with the blocking agent (BSA 2% (m/V), 30 min for the immunosensor and EA [1M], 10 min for the magnetic assay). The optimized assay consisted of the following steps: after the washing step, a previously prepared mixture (5 min before use) containing the Ab-D (2 µg/mL), HER2-ECD and BSA 0.5% (m/V) was added and left to incubate for 30 min. The washing step was then repeated, and an aliquot of an S-AP solution (5 × 10
−10 M) was added for 60 min. Subsequently, a washing step was carried out before proceeding. For the electrochemical measurements, a 40-µL aliquot of the mixture containing the enzymatic substrate (3-IP, 1.0 × 10
−3 M) and silver nitrate (4.0 × 10
−4 M) was placed on the SPCE and after 20 min the electrochemical signal was obtained by LSV using the following parameters: potential range: −0.03 V–+0.4 V; scan rate: 50 mV/s.
3. Results
3.1. Sensor Optimization
The antibody concentrations were optimized (Ab-C 10 and 25 µg/mL and Ab-D 0.5, 1 and 2 µg/mL), and the combination of Ab-C 25 µg/mL and Ab-D 2 µg/mL was selected to proceed the study.
The incubation time of the mixture HER2-ECD + Ab-D (containing 0.5% BSA) was studied using: (t1) 30 minutes and (t2) 60 min. Three concentrations of S-AP were tested: S-AP1 (2 × 10−10 M); S-AP2 (5 × 10−10 M) and S-AP3 (1 × 10−9 M), using two incubation times: t3 (30 min) and t4 (60 min). These parameters were tested with the previously optimized antibody concentrations.
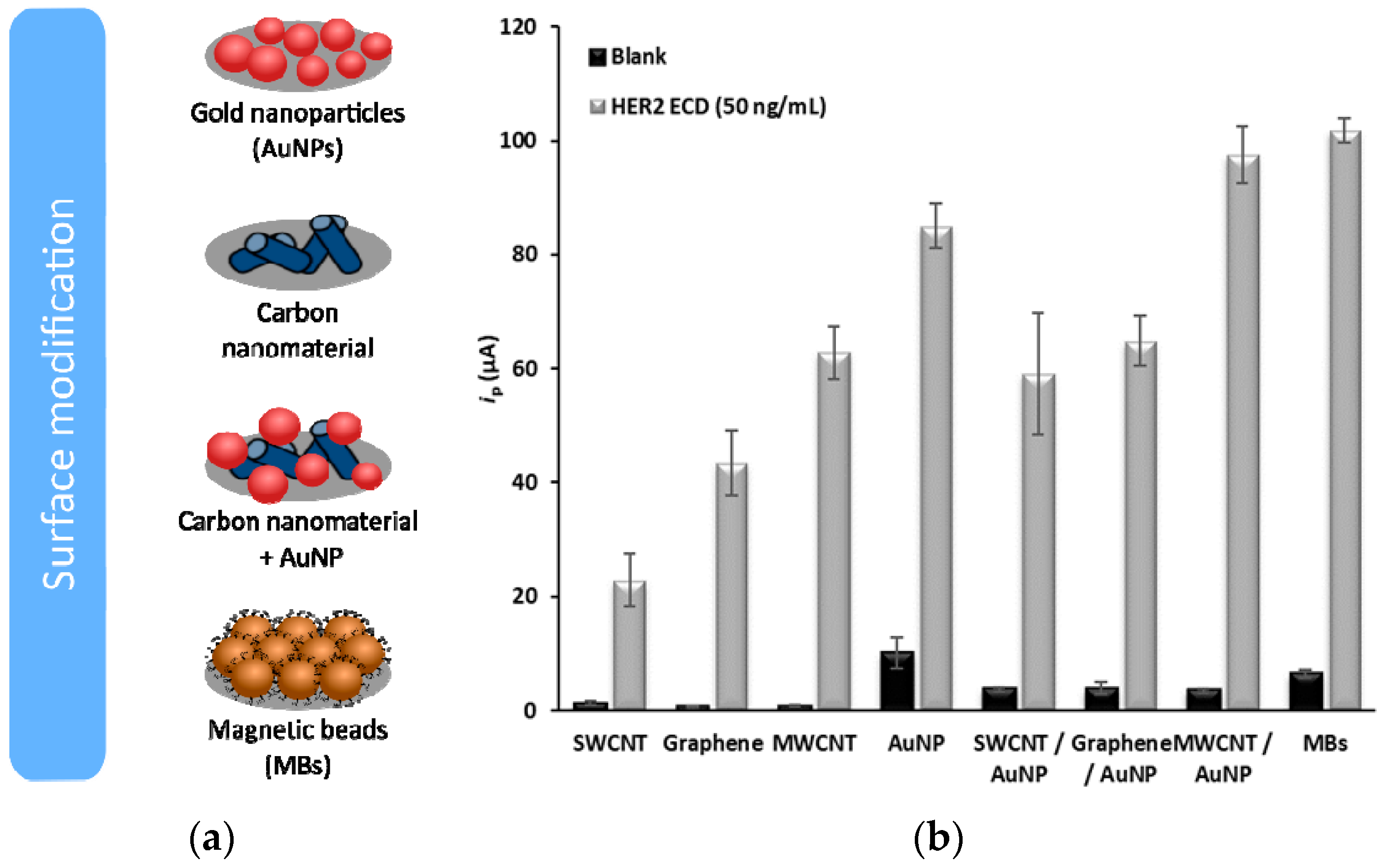
The results obtained with the distinct sensing platforms are presented in
Figure 1.
The analytical responses toward different biomarker concentrations using the best surface modifications (AuNP and MWCNT/AuNP) and for the magnetic immunoassay (MBs) were firstly tested in buffer. Then, male human serum samples were spiked with HER2-ECD and evaluated in the range of 7.5–100 ng/mL (
Figure 2a).
Selectivity studies were performed through analysis of possible serum interferents: human serum albumin (HSA, 35 mg/mL), cancer antigen 15-3 (CA 15-3, 30 U/mL) and cystatin C (cyst C, 565 ng/mL). The current intensities obtained for these non-target proteins are shown in
Figure 2b.
4. Discussion
The distinct sensing surface platforms were studied, and the signals obtained using a 50-ng/mL HER2-ECD solution were compared. Between the two carbon nanotube types, either individually or combined with AuNPs, the use of MWCNTs led to the highest signal. However, only when the MWCNT-AuNP combination was used an increase of the signal compared with the SPCE-AuNP was observed. HER2-ECD could also be analyzed using the MBs, but with a lower sensitivity than with the (modified) SPCEs.
The different sensing strategies were tested in spiked human serum samples. Linear relationships between ip and [HER2 ECD] (7.5–50 ng/mL) were established. The limits of detection (LOD) were calculated from the respective calibration plots (presented in
Figure 2a), obtaining LODs of 8.5 ng/mL for the SPCE-AuNP, 0.16 ng/mL for the SPCE-MWCNT/AuNP and 2.8 ng/mL for the magnetic assay.
The results of the selectivity studies (
Figure 2b) show that the immunomagnetic assay was the most selective, which may result from the efficient washing steps; a great advantage in the use of magnetic bioassays. Nevertheless, in the other sensing strategies, an excellent surface coverage (confirmed by SEM analysis) was obtained, and the results also confirmed the great selectivity of these platforms.
Recovery studies indicated that accurate results were obtained. It was verified that the nano- and micro material-based electrochemical bioassays for the non-invasive detection of HER2-ECD enhances the detection of the biomarker under study, improving the LOD and allowing the development of more precise analytical methodologies, not only in the early detection but also in the follow-up.
5. Conclusions
The best results were obtained for the SPCE-MWCNT/AuNP and SPCE-MBs using Ab-C 25 µg/mL and Ab-D 2 µg/mL. The total assay time was 2h20min. Spiked human serum samples were used to test the sensor’s applicability and the selectivity was confirmed through the analysis of other biomarkers and possible serum interferents.
Author Contributions
M.F. (investigation: Equal; Methodology: Equal; Writing—original draft: Lead); H.P.A.N. (Conceptualization: Lead; Funding acquisition: Equal; Methodology: Lead; Project administration: Equal; Supervision: Equal; Validation: Equal; Writing—review & editing: Equal); C.D.-M. (Funding acquisition: Equal; Methodology: Equal; Resources: Lead; Supervision: Equal; Validation: Equal; Writing—review & editing: Supporting).
Funding
Maria Freitas is grateful to FCT—Fundação para a Ciência e a Tecnologia for her PhD grant (SFRH/BD/111942/2015), financed by POPH-QREN-Tipologia 4.1-Formação Avançada, subsidized by Fundo Social Europeu and Ministério da Ciência, Tecnologia e Ensino Superior. This work received financial support from the European Union (FEDER funds through COMPETE) and National Funds (FCT) through project UID/QUI/50006/2019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Ponti, A.; Anttila, A.; Ronco, G.; Senore, C.; Basu, P.; Segnan, N.; Tomatis, M.; Žakelj, M.P.; Dillner, J.; Fernan, M.; et al. Cancer Screening in the European Union (2017): Report on the Implementation of the Council Recommendation on Cancer Screening (Second Report); European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Martínez-Paredes, G.; González-García, M.B.; Costa-García, A. In situ electrochemical generation of gold nanostructured screen-printed carbon electrodes. Application to the detection of lead underpotential deposition. Electrochim. Acta 2009, 54, 4801–4808. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).