Cobalt-Dispersed Reduced Graphene Oxide Nanocomposite for the Selective lectrochemical Detection of Methyl Nicotinate †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
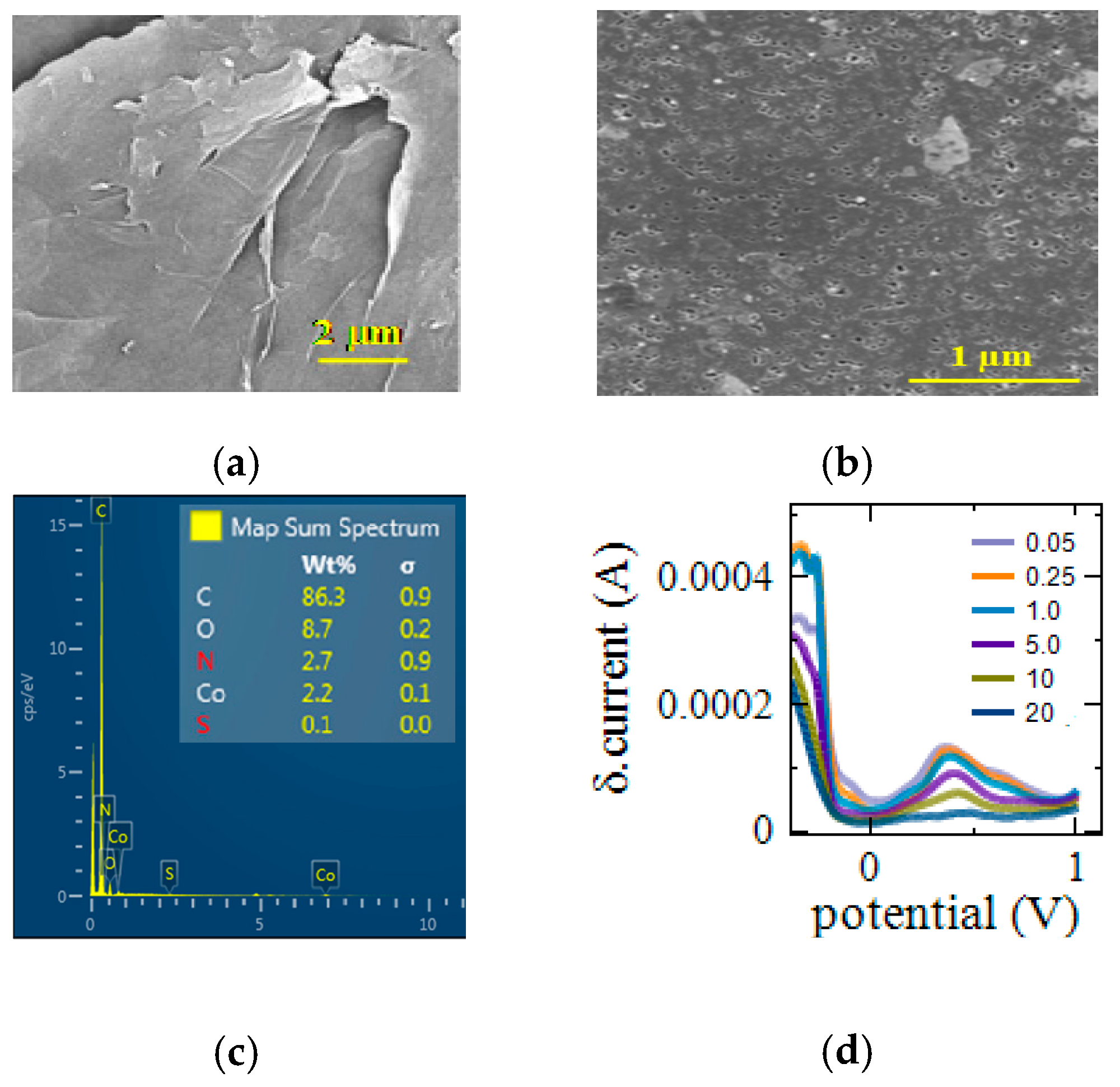
3.1. Surface Morphology
3.2. Electrochemical Characterization and Measurements
3.3. DPV Measurements
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Abdelwahab, A.E. Immonologocal and molecular diagnosis of mycobacterium tuberculosis between two enviromentally different regions. Aced. J. Inc. 2009, 1, 1–8. [Google Scholar]
- Li, L.; Yuan, Y.; Chen, Y.; Zhang, P.; Bai, Y.; Bai, L. Aptamer based voltammetric biosensor for Mycobacterium tuberculosis antigen ESAT-6 using a nanohybrid material composed of reduced graphene oxide and a metal-organic framework. Microchim. Acta 2018, 185, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Smith, Y.R.; Mishra, M.; Mohanty, S.K. Electrochemical detection of methyl nicotinate biomarker using functionalized anodized titania nanotube arrays. Mater. Res. Express 2015, 2, 025002. [Google Scholar] [CrossRef]
- Bairagi, P.K.; Gupta, G.S.; Verma, N. Fe-enriched clay-coated and reduced graphene oxide-modified N-doped polymer nanocomposite: A natural recognition element-based sensing electrode for DNT. Electroanalysis 2018, 30, 535–544. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Khare, P.; Ramkumar, J.; Verma, N. Carbon nanofiber-skinned three dimensional Ni/carbon micropillars: High performance electrodes of a microbial fuel cell. Electrochim. Acta 2016, 219, 88–98. [Google Scholar] [CrossRef]
| Electrodes | Rs (Ω) | Rct (Ω) | Potentials for MN Measurements | |
|---|---|---|---|---|
| Cathodic (V) | Anodic (V) | |||
| P*C | ~23 | ~17 | absent | −0.55 |
| Co-rGO/PC | ~20 | ~10 | 0.12 | −0.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
KumarBairagi, P.; Goyal, A.; NishithVerma. Cobalt-Dispersed Reduced Graphene Oxide Nanocomposite for the Selective lectrochemical Detection of Methyl Nicotinate. Proceedings 2019, 15, 18. https://doi.org/10.3390/proceedings2019015018
KumarBairagi P, Goyal A, NishithVerma. Cobalt-Dispersed Reduced Graphene Oxide Nanocomposite for the Selective lectrochemical Detection of Methyl Nicotinate. Proceedings. 2019; 15(1):18. https://doi.org/10.3390/proceedings2019015018
Chicago/Turabian StyleKumarBairagi, Pallab, Arpit Goyal, and NishithVerma. 2019. "Cobalt-Dispersed Reduced Graphene Oxide Nanocomposite for the Selective lectrochemical Detection of Methyl Nicotinate" Proceedings 15, no. 1: 18. https://doi.org/10.3390/proceedings2019015018
APA StyleKumarBairagi, P., Goyal, A., & NishithVerma. (2019). Cobalt-Dispersed Reduced Graphene Oxide Nanocomposite for the Selective lectrochemical Detection of Methyl Nicotinate. Proceedings, 15(1), 18. https://doi.org/10.3390/proceedings2019015018





