Nanocrystalline LaCoO3 Modified by Ag Nanoparticles with Improved Sensitivity to H2S †
Summary
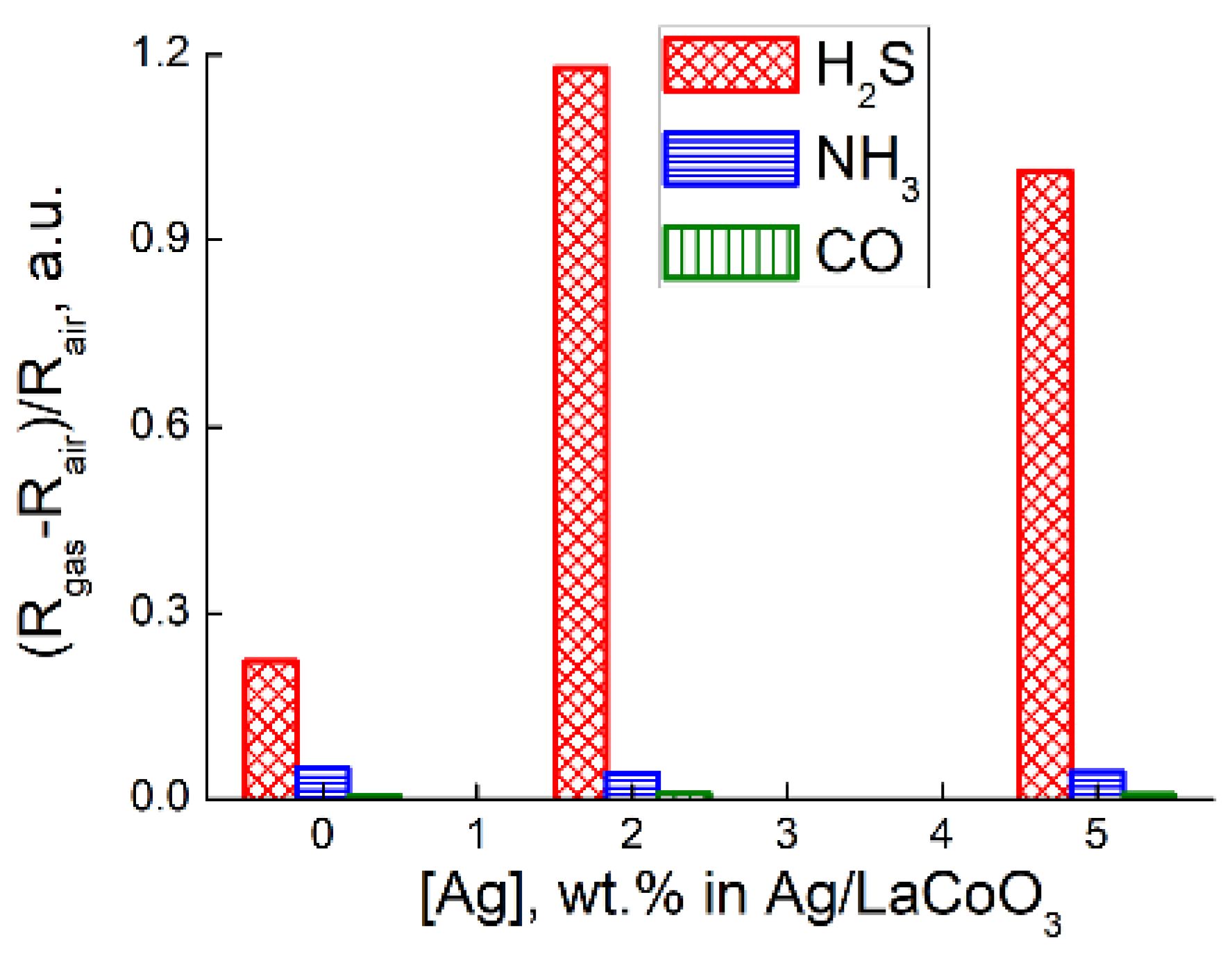
Motivation and Results
Funding
Acknowledgments
Conflicts of Interest



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marikutsa, A.; Chumakova, V.; Rumyantseva, M.; Gaskov, A. Nanocrystalline LaCoO3 Modified by Ag Nanoparticles with Improved Sensitivity to H2S. Proceedings 2019, 14, 44. https://doi.org/10.3390/proceedings2019014044
Marikutsa A, Chumakova V, Rumyantseva M, Gaskov A. Nanocrystalline LaCoO3 Modified by Ag Nanoparticles with Improved Sensitivity to H2S. Proceedings. 2019; 14(1):44. https://doi.org/10.3390/proceedings2019014044
Chicago/Turabian StyleMarikutsa, Artem, Valentina Chumakova, Marina Rumyantseva, and Alexander Gaskov. 2019. "Nanocrystalline LaCoO3 Modified by Ag Nanoparticles with Improved Sensitivity to H2S" Proceedings 14, no. 1: 44. https://doi.org/10.3390/proceedings2019014044
APA StyleMarikutsa, A., Chumakova, V., Rumyantseva, M., & Gaskov, A. (2019). Nanocrystalline LaCoO3 Modified by Ag Nanoparticles with Improved Sensitivity to H2S. Proceedings, 14(1), 44. https://doi.org/10.3390/proceedings2019014044





