Chemically Sensitive Photoluminescence of InGaN/GaN Nanowire Heterostructure Arrays †
1. Background
2. Experiment
3. Results
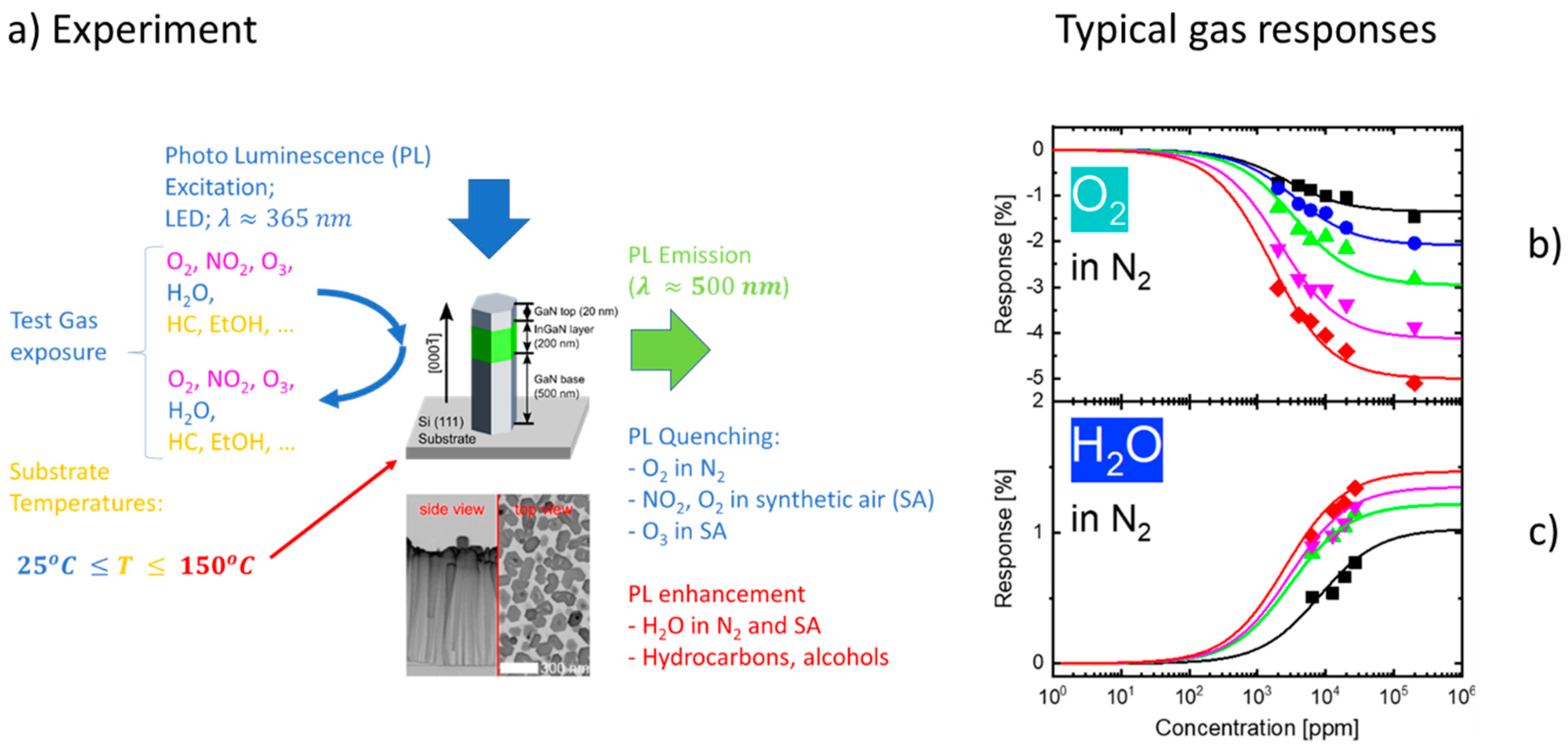
- (i)
- (ii)
- (iii)
Funding
Acknowledgments
Conflicts of Interest
References
- Fletcher, A.S.A.; Nirmal, D. A survey of Gallium Nitride HEMT for RF and high power applications. Superlattices Microstruct. 2017, 109, 519–537. [Google Scholar] [CrossRef]
- Chao, E. Analysis of LED technologies for solid state lighting markets. Technical report No. UCB/EECS-2012-138, EECS Department, University of California, Berkeley: Berkeley, CA, USA, 2012. Available online: https://www2.eecs.berkeley.edu/Pubs/TechRpts/2012/EECS-2012-138.html (accessed on 18 June 2018).
- Pearton, S.J.; Kang, B.S.; Kim, S.; Ren, F.; Gila, B.P.; Abernathy, C.R.; Lin, J.; Chu, S.N.G. GaN-based diodes and transistors for chemical, gas, biological and pressure sensing. J. Phys. Condens. Matter. 2004, 16, R961–R994. [Google Scholar] [CrossRef]
- Shahbaz, J.; Schneidereit, M.; Thonke, K.; Scholz, F. Functionalized GaN/GaInN heterostructures for hydrogen sulfide sensing. Jap. J. Appl. Phys. 2019, 58, Number SC. [Google Scholar] [CrossRef]
- Winnerl, J.; Hudeczek, R.; Stutzmann, M. Optical Design of GaN Nanowire Arrays for Photocatalytic Applications. J. Appl. Phys. 2018, 123, 203104. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Müller, G.; Becker, P.; Hille, P.; Schörmann, J.; Teubert, J.; Eickhoff, M. Detection of Oxidising Gases Using an Optochemical Sensor System Based on GaN/InGaN Nanowires. Sens. Actuators B Chem. 2014, 197, 87–94. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Müller, G.; Hille, P.; Teubert, J.; Eickhoff, M. Photoluminescence Probing of Complex H2O Adsorption on InGaN/GaN Nanowires. Nano Lett. 2017, 17, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Maier, K.; Helwig, A.; Müller, G.; Eickhoff, M. Photoluminescence Detection of Surface Oxidation Processes on InGaN/GaN Nanowire Arrays. ACS Sens. 2018, 3, 2254–2260. [Google Scholar]
- Maier, K.; Helwig, A.; Müller, G.; Hille, P.; Teubert, J.; Eickhoff, M. Competitive Adsorption of Air Constituents as Observed on InGaN/GaN Nano-Optical Probes. Sens. Actuators B Chem. 2017, 250, 91–99. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Müller, G.; Eickhoff, M. Luminescence Probing of Surface Adsorption Processes Using InGaN/GaN Nanowire Heterostructure Arrays. In Semiconductor Gas Sensors; Woodhead Publishing: Cambridge, UK, 2019; in press. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maier, K.; Helwig, A.; Müller, G.; Eickhoff, M. Chemically Sensitive Photoluminescence of InGaN/GaN Nanowire Heterostructure Arrays. Proceedings 2019, 14, 43. https://doi.org/10.3390/proceedings2019014043
Maier K, Helwig A, Müller G, Eickhoff M. Chemically Sensitive Photoluminescence of InGaN/GaN Nanowire Heterostructure Arrays. Proceedings. 2019; 14(1):43. https://doi.org/10.3390/proceedings2019014043
Chicago/Turabian StyleMaier, Konrad, Andreas Helwig, Gerhard Müller, and Martin Eickhoff. 2019. "Chemically Sensitive Photoluminescence of InGaN/GaN Nanowire Heterostructure Arrays" Proceedings 14, no. 1: 43. https://doi.org/10.3390/proceedings2019014043
APA StyleMaier, K., Helwig, A., Müller, G., & Eickhoff, M. (2019). Chemically Sensitive Photoluminescence of InGaN/GaN Nanowire Heterostructure Arrays. Proceedings, 14(1), 43. https://doi.org/10.3390/proceedings2019014043



