Industrial pollution and traffic emissions emit dangerous amounts of O3, NO2, VOCs and PM into environment, bringing higher incidence of morbidity and mortality in respiratory sicknesses [1]. Among tropospheric pollutant species, monitoring the O3 concentration is remarkably important for its toxicity. The aftereffects of O3 exposure indeed are upper respiratory irritation, rhinitis, cough, headache, occasional nausea, and vomiting [2]. In 2015, the United States Environmental Protection Agency (EPA), reinforced the National Ambient Air Quality Standards (NAAQS) for O3 at ground-level not to exceed 70 ppb to improve the protection of human health [3].
This work presents n-type In2O3 as sensitive material to detect O3 between 0.1 and 1 ppm at low temperatures (75 °C–150 °C). In2O3 powders were synthetized by hydrothermal route [4], with the goal to achieve a finer crystallite size, higher specific surface area and lower degree of agglomeration compared to commercial In2O3 (Sigma Aldrich, St. Louis, MO, USA). Those characteristics are essential to enhance the sensor performances [5].
In the synthesis, In2O3 nanostructures were realized by hydrothermal method using indium nitrate as indium precursor, soda as mineralizer and CTAB as capping agent, according to previous literature [6]. The mixture was maintained for 24 h at 70 °C and then for 12 h at 120 °C. Subsequently, powders were calcined at 400 °C for 30 min obtaining In2O3 [4].
In2O3 powders were characterized by laser granulometry, Thermal Analysis, X-ray Diffraction, N2 adsorption, Field Emission-Scanning Electron Microscopy and High-Resolution Transmission Electron Microscopy.
Sensors were fabricated by screen-printing technique onto α-alumina substrates with Pt electrodes and a backside Pt heater. Inks for screen-printing were realized by mixing In2O3 powders with ethylene glycol monobutyral ether (Emflow), as organic vehicle and polyvinyl butyral (PVB) acting as temporary binder. After screen-printing deposition sensors were dried at 80 °C overnight and fired at 500 °C for 1 h in air. For obtaining different layer thicknesses in the range 10–100 µm, the first layer was dried and then a new layer was printed onto it.
Films were characterized by Scanning Electron Microscopy, electrical measurements and operando diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy.
Sensors were tested towards different amounts of O3, NO2 and H2 under 0, 30% and 60% of RH (relative humidity) to study the selectivity of the as-realized chemical sensors for O3 detection. Best results were achieved at 150 °C towards O3, with the sensor selectivity for O3 increasing by increasing the working temperature from 75 °C to 150 °C. Both oxidant gases (O3 and NO2) showed best performance at higher RH amounts, whereas for H2 the trend was opposite, probably due to the competition between H2 and H2O for the same adsorption sites on the In2O3 surface. At 150 °C, under 1 ppm O3, the variation of film resistance is 5 orders of magnitude, while it was only equal to 2 orders of magnitude under 1 ppm of NO2. Under 30% RH, the influence of sensor thickness is much higher under O3 compared to NO2 and a logical trend was noticed in which by changing one order of magnitude the sensor thickness, the sensor response varies of more than 3 orders of magnitude under 1 ppm O3. Under NO2, only a small influence of the sensing film thickness on the sensor response was detected. Finally, the interference with H2 is negligible as the sensor response towards H2 is independent from the film thickness, as expected. Calibration curves of In2O3 sensor towards O3, NO2 and H2 at 150 °C and 30% RH in the range 10–100 µm of thickness are displayed in Figure 1.
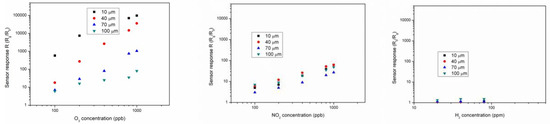
Figure 1.
Comparison between 10, 40, 70 and 100 µm thick In2O3 sensor response towards O3 (left), NO2 (center) and H2 (right) at 150 °C and 30% RH.
By DRIFTS, the aim is to clarify the interaction of NO2 and O3 with In2O3 surface establishing tightly the relationship between surface structure and adsorbed species with the gas sensing response.
Considering the NO2-In2O3 interaction, the OH groups, most likely due to adsorbed water on the In2O3 surface, play a key role in the NO2 adsorption. NO2 withdraw more electrons from In2O3 in the presence of water forming nitrites and resulting in the measured increased in electrical resistance. This is confirmed by the higher increase in electrical resistance under humid atmospheres. OH groups are consumed when NO2 is adsorbed onto the surface in the form of nitrites and in this process, H bonds are broken.
In the interaction of O3 with In2O3 surface, signals related to peroxide formation during O3 adsorption and decomposition were detected as well as peaks due to physisorbed O3 still present at 150 °C. Furthermore, bands generated by carbonate-like species formed through reactions of O3 with residual carbonaceous impurities from the synthesis route were recognized.
To conclude, in this work the role of the film thickness under O3, NO2 and H2 exposure was studied for In2O3 sensor realized by screen printing technique. Finally, by operando DRIFT a complex sensing mechanism has been evidenced for In2O3 sensors, involving OH groups and adsorbed water in the mechanism of NO2 adsorption and peroxide formation and O3 physisorption during O3 exposure.
References
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water—A Practical Guide to Understanding Ozone and Its Applications; Wiley: Weinheim, Germany, 2000. [Google Scholar]
- 2015 National Ambient Air Quality Standards (NAAQS) for Ozone. Available online: https://www.epa.gov/ozone-pollution/2015-national-ambient-air-quality-standards-naaqs-ozone (accessed on 7 April 2019).
- Shinde, D.V.; Ahn, D.Y.; Jadhav, V.V.; Lee, D.Y.; Shrestha, N.K.; Lee, J.K.; Lee, H.Y.; Maneb, R.S.; Han, S.H. A coordination chemistry approach for shape controlled synthesis of indium oxide nanostructures and their photoelectrochemical properties. J. Mater. Chem. A 2014, 2, 5490–5498. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cerneavschi, A.; Ivanov, M.; Golovanov, V.; Cornet, A.; Morante, J.; Cabot, A.; Arbiol, J. The influence of film structure on In2O3 gas response. Thin Solid Films 2004, 460, 315–323. [Google Scholar] [CrossRef]
- Yan, T.; Wang, X.; Long, J.; Liu, P.; Fu, X.; Zhang, G.; Fu, X. Urea-based hydrothermal growth, optical and photocatalytic properties of single-crystalline In(OH)3 nanocubes. J. Colloid Interface Sci. 2008, 325, 425–431. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).