Abstract
This study examines the significance of thermodynamic model selection to improve predictions when modeling a wet oxidation (WO) process. WO is a promising technology for treating the highly concentrated process water stream from hydrothermal liquefaction (HTL) while generating heat, due to the exothermic oxidation reactions, leading to a potential integrated HTL-WO autothermal process. However, the harsh process conditions employed fail to describe oxygen solubility accurately, leading to major deviations in predicted COD reduction, heat generation, vapor fraction, and final design. To accurately capture oxygen solubility at elevated temperatures and pressures, experimental oxygen solubility data were regressed using activity coefficient models. This yielded improved oxygen solubility predictions at 280–350 °C, more realistic vapor fractions and heat outputs, and COD reduction close to experimental values.
1. Introduction
Hydrothermal liquefaction (HTL) converts biomass into biocrude oil, but treating the highly concentrated resulting process water (HTL-PW) is a major challenge for the further development of the technology. Wet oxidation (WO) has been shown to oxidize organic at up to 350 °C and 220 bar, achieving up to 85% COD removal in HTL-PW within 15 min [1]. Process modeling is vital for scaling up WO, allowing analysis of mass and energy balances, heat release, equipment sizing and potential for heat integration with HTL. Accurate thermodynamic models are key to capturing the non-ideal behavior of water, oxygen, hydrocarbons, and gases across varying temperatures and pressures. Previously [2,3,4], WO has been modeled by the Predictive Soave–Redlich–Kwong (PSRK) Equation of State (EOS) due to its predictive nature and the elevated temperatures and high pressures employed. Zhang et al. [3] found that PSRK showed the least deviation in oxygen solubility from experimental data out of all EOSs tried; however, they did not examine temperatures higher than 180 °C. Schuck et al. [2] used PSRK both for the validation of a kinetic process model in batch scale and for the scale up to industrial relevant conditions. However, the low COD concentration of the HTL-PW considered in the study (28.5 g/L) used only 3% w/w oxygen, avoiding unusual behavior like high vapor fractions or low residence times in the upscaled design. Nevertheless, industrial HTL applications have higher COD levels due to increased dry matter in feed slurries. Higher HTL-PW COD concentrations would require more oxygen for the complete oxidation (e.g., 10% w/w for 100 g/L COD). Under these conditions, EOS such as PSRK fail to describe oxygen solubility at temperatures higher than 280 °C, giving substantial differences in the predicted COD reduction, heat generation, vapor fraction, residence time and hence the final design. This study evaluates thermodynamic models at elevated temperatures to improve WO modeling and support HTL integration and scale-up.
2. Methodology
Several thermodynamic property models were tested for oxygen solubility in water, including PSRK, Peng–Robinson, and Volume-Translated Peng–Robinson (VTPR). Two versions of the electrolyte non-random two-liquid (ENRTL) model were also tested: ENRTL-HF, which uses the hydrogen fluoride equation of state for low-pressure vapor-phase, and ENRTL-HG, which applies the Helgeson model for high-temperature and high-pressure calculations. Additionally, regression analysis was performed to refine the UNIQUAC–Hayden–O’Connell (UNIQ-HOC) model using experimental oxygen solubility data from Baranenko et al. [5]. A T-xy dataset was created in Aspen Plus at 200 bar, incorporating standard deviations of 0.05 °C for temperature and 0.1% for oxygen mole fractions. Regression with and without Henry’s constants used initial Bij = 255.002, Bji = −376.691, and scale factor 1. The binary interaction parameters obtained from both regressions were applied to Aspen Plus, forming the refined ‘UNIQ-HOC Reg’ model. Property model evaluations and process simulations were conducted using steady-state models in Aspen Plus V12®. To study O2 solubility in water, two streams—one with 1000 kg/h of water and the other with varying O2 flow rates—were combined in a mixer block at 20 °C and 1 bar. The mixture was heated and pressurized to 200 bar, then flashed at 0 bar to analyze O2 solubility. A sensitivity analysis assessed solubility across temperatures from 0 to 360 °C. Following the O2-H2O evaluation, the UNIQ-HOC and ENRTL models were applied to a WO simulation model previously developed [2]. The HTL-PW composition reflected a large-scale plant producing 1260 kg/h with a COD of 108,000 mg/L and TN of 8300 mg/L (half considered as ammonia). The HTL-PW was pressurized to 220 bar, heated to 250 °C, and mixed with 126 kg/h of compressed O2 (stoichiometric oxygen for complete oxidation). The mixture entered a multitube adiabatic plug flow reactor (PFR), and the outlet stream was cooled and flashed at 40 °C and 1 bar. PENG-ROB was the selected thermodynamic model for the oxygen compressor, while the different variations of ENRTL and UNIQ-HOC were used for the remaining blocks.
3. Results and Discussion
Over ten high-pressure models were tested, but none captured oxygen solubility well at high temperatures. As shown in Figure 1, PSRK provided reliable solubility predictions up to 260 °C across all tested O2:H2O ratios, outperforming PR and VTPR models. At 300 °C and 3 wt.% O2, PSRK overestimates solubility (0.94 mol/kg vs. 0.37 mol/kg experimentally) and fails to capture the decreasing trend above 300 °C, inaccurately predicting all components in the liquid phase. For higher O2:H2O ratios relevant to industrial settings, PSRK predicts a vapor-only phase at 300 °C, with solubility dropping to zero, then rising anomalously at higher temperatures. At 350 °C and 10 wt.% O2, it predicts full oxygen solubility, and at 20 wt.% O2, solubility is six times higher than experimental values at 360 °C. This highlights PSRK’s limitations at 300–350 °C, crucial for WO of HTL-PW, despite its frequent use in WO studies [3,4].
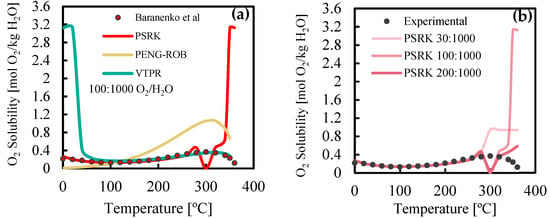
Figure 1.
(a) Evaluation of different models for solubility of O2 in water at 200 bar and (b) at different O2:H2O ratios, compared with experimental results by Baranenko et al. [5].
Simulations were conducted to assess the oxygen solubility in water using the ENRTL-HG and ENRTL-HF models, as well as experimental data [5] that was regressed with the UNIQ-HOC activity coefficient models. Figure 2a compares the performance of ENRTL-HG, ENRTL-HF, and two UNIQ-HOC Reg models (with and without Henry’s constant). While all models deviate from experimental data below 20 °C, these temperatures are irrelevant for WO operation temperatures. As shown in Figure 2b, solubility remains unaffected by the oxygen-to-water ratio within the tested range due to constant partial pressure. Solubility decreases above 300 °C, reaching its lowest point at 360 °C. Given the limited data on O2 solubility, further verification of properties like density, organics’ solubility, and phase equilibria in HTL-PW would be valuable.
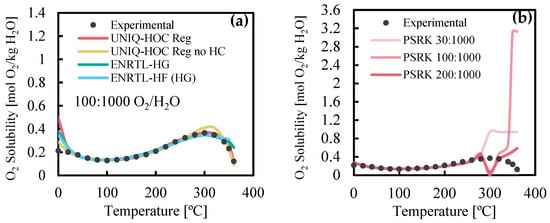
Figure 2.
(a) Evaluation of regressed models for solubility of O2 in water at 200 bar and (b) regressed UNIQ-HOC at different O2:H2O ratios, compared with experimental results by Baranenko et al. [5].
The predicted oxygen solubility from regressed data aligns well with experimental findings [5] and was further evaluated in a WO system for organic compound oxidation at elevated temperatures and pressures. A sensitivity analysis was conducted by varying the reactor residence time to assess the impact of the regressed models on key variables. Figure 3a illustrates COD reduction trends for different models over varying residence times. As expected, COD decreases with increased residence time. ENRTL-HF (HG) shows the lowest reduction, while UNIQ-HOC Reg achieves the highest, reaching approximately 15 g/L (85% reduction) after 170 min. All models exhibit a steep initial drop, with significant organic oxidation occurring within the first four minutes. Most models agree on the magnitude of this drop, except for ENRTL-HF (HG), which shows a smaller decrease. Figure 3b illustrates the temperature profile for different models over varying residence times. A steep temperature rise is observed during the initial reactor operation, reflecting heat generation from oxidation reactions and corresponding COD reduction. Subsequently, the temperature increases gradually, approaching a plateau near 360 °C. This results from rising heat capacity and reduced oxidation. The vapor fractions for the models, shown in Figure 3c, generally align with the corresponding COD removal and temperature profiles. However, the ENRTL-HG model exhibits an unexpectedly high vapor fraction that does not correspond to its extended residence time. Figure 3d compares acetic acid conversion with batch data from Silva et al. at 350 °C [6], highlighting noticeable differences between the models’ predictions. Discrepancies stem from batch data being preheated to 350 °C, while simulations heat from 250 °C, slowing early conversion. However, all models align well with experimental observations of acetic acid formation within the first 10–15 min, followed by a decline in concentration.
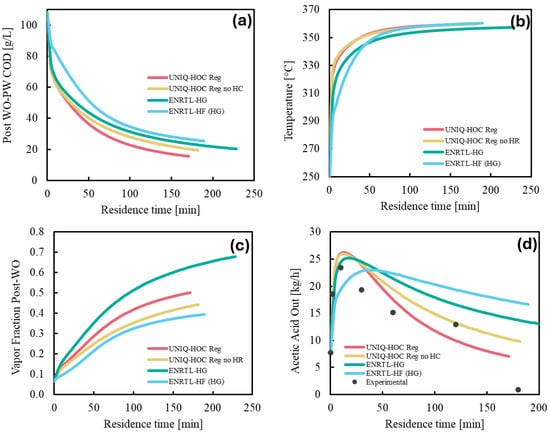
Figure 3.
Influence of the selected property and regressed model at varying residence time on the (a) COD content of post-WO, (b) predicted WO outlet temperature, (c) vapor fraction in the WO outlet stream, and (d) acetic acid concentration (experimental data from [6]).
4. Conclusions
This study highlights the critical role of thermodynamic model selection for accurately simulating the wet oxidation (WO) process at elevated conditions. Regression of experimental oxygen solubility data using activity coefficient models, coupled with an EoS for the vapor phase, significantly improved prediction accuracy for key process parameters. The UNIQ-HOC Regressed model performed best and is recommended.
Author Contributions
Writing—original draft preparation, formal analysis, validation, A.H.; Software, validation, formal analysis, visualization, B.T.H.S.; Conseptualization, methodology, writing—review and editing, supervision, project administration, K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Silva Thomsen, L.B.; Anastasakis, K.; Biller, P. Hydrothermal liquefaction potential of wastewater treatment sludges: Effect of wastewater treatment plant and sludge nature on products distribution. Fuel 2024, 355, 129525. [Google Scholar] [CrossRef]
- Schuck, C.E.; Schäfer, T.; Anastasakis, K. Predictive Modeling and scale-up of Wet Oxidation for Hydrothermal Liquefaction Process Water treatment. Comput. Aided Chem. 2023, 52, 2229–2234. [Google Scholar]
- Zhang, F.; Chen, J.; Su, C.; Ma, C. Energy Consumption and Economic Analyses of a Supercritical Water Oxidation System with Oxygen Recovery. Processes 2018, 6, 224. [Google Scholar] [CrossRef]
- Lefèvre, S.; Boutin, O.; Ferrasse, J.H.; Malleret, L.; Faucherand, R.; Viand, A. Thermodynamic and kinetic study of phenol degradation by a non-catalytic wet air oxidation process. Chemosphere 2011, 84, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Baranenko, V.I.; Fal’kovskii, L.N.; Kirov, V.S.; Kurnyk, L.N.; Musienko, A.N.; Piontkovskii, A.I. Solubility of oxygen and carbon dioxide in water. Soviet Atomic Energy. 1990, 68, 342–346. [Google Scholar] [CrossRef]
- Silva Thomsen, L.B.; Anastasakis, K.; Biller, P. Wet oxidation of aqueous phase from hydrothermal liquefaction of sewage sludge. Water Res. 2022, 209, 117863. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).