1. Introduction
Ornamental stones such as granite and marble are economically important worldwide, but their processing generates large volumes of waste, mainly stone dust and water, which poses environmental concerns due to its often improper disposal in nature [
1]. Even when reused in construction, the excessive amount of waste produced calls for alternative recycling methods [
2,
3]. Thus, the reuse of Ornamental Stone Waste (OSW) as a substitute for bentonite in drilling fluids is a promising approach, due to its mineral compatibility with carbonate rocks.
To enhance particle stability in solution, surface functionalization can modify surface interactions with the continuous phase and suspension properties. Terephthalic acid (TPA) is an organic acid that contains two deprotonable carboxylate groups, making it highly effective for surface modification as a preparatory step for reactions with polymer chains. TPA acts as a “bridge”, with its carboxylic terminations connecting both the particle surface and the polymer [
4]. This allows for the combination of minerals with polymers such as xanthan gum (XG), one of the most used polymers in drilling fluids [
5], to improve their rheological properties.
Therefore, this paper explores the potential reuse of OSW in aqueous fluids, in combination with XG as an additive, as well as the surface functionalization of OSW particles with TPA to form a stable dispersion exhibiting shear-thinning behavior.
2. Method
OSW was supplied by the granite industry, oven-dried at 105 °C for 24 h, and characterized. Mineralogy was analyzed by means of X-ray diffraction (XRD-6000 Shimadzu, Kyoto, Japan, Cu-Kα, 5°–80°). Scanning electronic microscopy (SEM, Jeol JSM6610LV, Tokyo, Japan), coupled with an energy dispersive X-ray spectrophotometer (EDX, Bruker 6-10 detector, Karlshure, Germany), was used for morphological analysis. Gravimetric analysis was conducted in a muffle furnace (T One Plus, Guarulhos, Brazil) at a 10 °C min−1 heating rate up to 800 °C. The Fourier transform infrared (FTIR, 4000–650 cm−1) spectrum was recorded using a Cary 630 (Agilent, Santa Clara, CA, USA). Zeta potential was measured at 25 °C (Nanotrac Wave II–Microtrac, Montgomeryville, PA, USA) across varying pH values.
2.1. OSW Modification with TPA
OSW samples were treated with TPA (Sigma-aldrich–99.0%, Milwaukee, WI, USA) by reacting 2 g of OSW with 40 mL of 1% (w/w) TPA solution in deionized water (DW) at 100 °C for 4 h under magnetic stirring. The samples were washed with DW until reaching a constant pH, then dried at 80 °C for 24 h, and characterized using FTIR, zeta potential, and gravimetry.
2.2. Preparation of Water-Based Fluid with OSW and OSW-TPA
Aqueous fluids were prepared by hydrating XG in ultrapure water (1.5 gL−1) and leaving it to rest at 5 °C for 48 h. Then, the solution was subjected to high-shear mixing at 15,000 rpm for 10 min (Ultra Turrax IKA T25, Campinas, Brazil). Fluids were prepared at pH 7 with OSW/bentonite ratios of 0:100; 50:50; and 100:0, maintaining a 4.86% (w/w) solid concentration in the polymeric fluid.
After shearing the polymeric solution, mineral components (OSW, OSW-TPA, or bentonite) were added and mixed again for 10 min. Fluids were left to rest for 24 h before rheological testing.
2.3. Rheological Behavior
Rheological behavior was evaluated using a Haake Mars III rheometer (Thermo Scientific, Walthan, MA, USA) with CC25 DinTi geometry at 25 °C. Flow curves were obtained over a shear rate (
) range of 1–1000 s
−1, recording the shear stress (
). The Herschel–Bulkley (HB) model in Equation 1 [
6] was used to determine yield stress (
), consistency index (
), and flow behavior index (
) with R
2 as the model fit indicator.
3. Results and Discussion
3.1. OSW and OSW-TPA Characterization
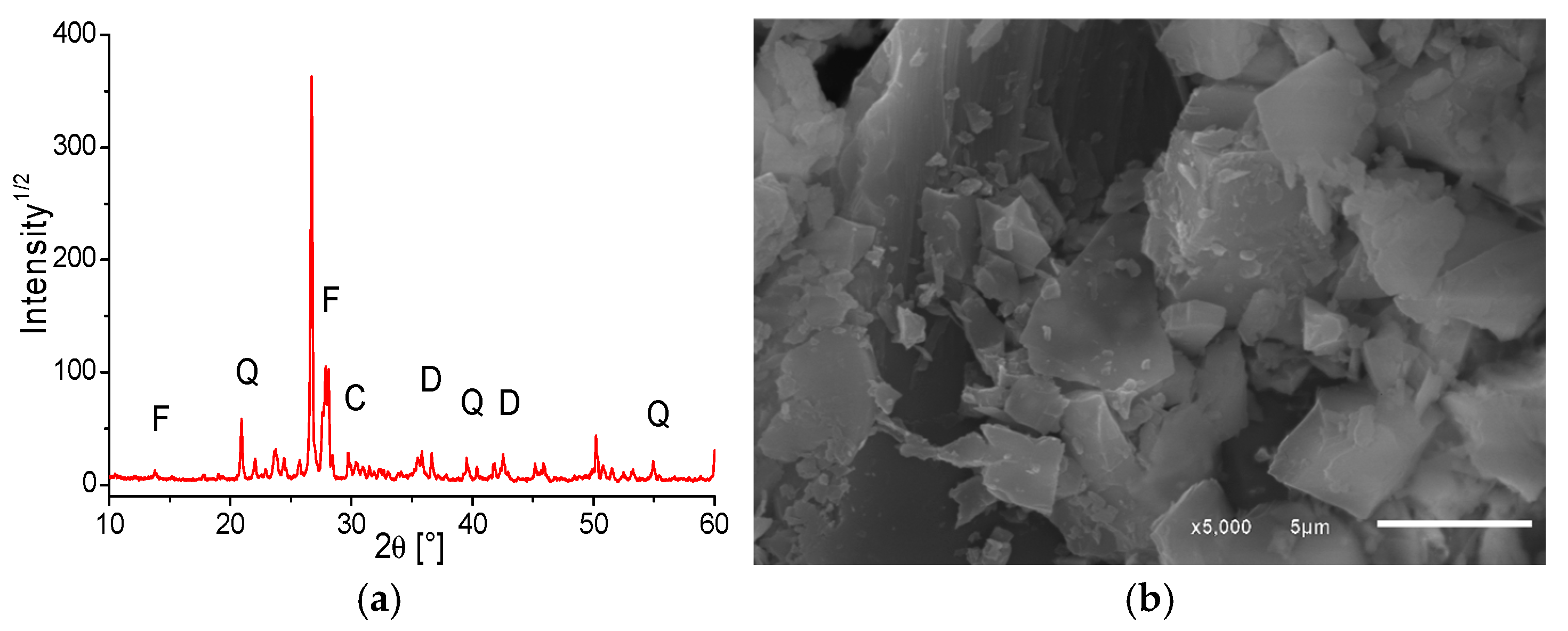
The XRD and SEM analyses of OSW (
Figure 1) revealed that the waste primarily consists of feldspar (F), quartz (Q), calcite (C), and dolomite (D), with an average particle size of 54.14 µm. The particles exhibit irregular shapes, varying sizes, and a disordered arrangement. EDX analysis showed the elements present on the surface of pure OSW particles, with the major components being oxygen (66.08%), silicon (14.57%), sodium (7.75%), aluminum (6.72%), and magnesium (3.13%).
Gravimetric analysis showed that pure OSW lost 1.8% of its weight, while OSW-TPA lost 18.36% after calcination, indicating a significantly higher organic matter content in OSW-TPA due to the TPA adsorbed on the OSW surface. The FTIR spectra in
Figure 2a reveal the appearance of a broad band between 3164 and 2266 cm
−1 in OSW-TPA, attributed to the O-H stretching of the carboxylic acid groups in TPA. The absorption peak at 1684 cm
−1 corresponds to the C=O stretching of the acid. The peaks at 1571 and 1507 cm
−1 are associated with C=C bonds of the aromatic ring present in the TPA structure. The most intense absorption peak at 965 cm
−1 is attributed to aluminosilicates. There is an overlap of absorptions at 777 cm
−1, which is related to SiO
2 bonds in quartz and aromatic ring vibrations [
4]. Thus, TPA may be bound to the mineral either through interactions between carboxylate groups and metals ions in the OSW structure or through physical adsorption on the OSW surface.
The zeta potential values shown in
Figure 2b indicate a predominance of positive surface charges at acidic pH levels and negative charges at basic pH levels for both pure OSW and OSW-TPA. The pH at which the surface charge is neutral (isoelectric point) was 6.56 for OSW-TPA and 8.31 for pure OSW, indicating that the suspensions exhibit the lowest stability at these points. Suspensions are considered stable when the electrostatic repulsive forces between particles exceed the van der Waals attractive forces, preventing agglomeration. This occurs when the zeta potential is equal to or greater than ± 30 mV [
4]. Therefore, the most stable suspensions were observed at pH values above 9 for OSW-TPA, whereas for pure OSW, stability was only observed at pH 11.
3.2. Rheological Characterization
The effects of physical interaction between XG and OSW or OSW-TPA on the behavior of aqueous fluids were analyzed through the flow curves in
Figure 3. A significant decrease in viscosity and shear stress was observed as the concentration of OSW and OSW-TPA increased at the expense of bentonite. At a 50% concentration, OSW-TPA exhibited behavior similar to that of fluids with OSW; however, at a 100% concentration, OSW-TPA resulted in significantly inferior rheological properties.
The rheological parameters obtained from HB model fitting are presented in
Table 1. All fluids were statistically well-fitted by the HB model (R
2 ≈ 1). The
and
values increased with bentonite concentration, indicating that the addition of waste materials does not enhance either viscosity or yield stress. The
value for most fluids was approximately 0.5. Values of
below 1 indicate shear-thinning behavior. Therefore, most fluids exhibited pronounced shear-thinning behavior, except for the OSW-TPA 100% fluid. The viscosity of this last fluid was close to that of water, suggesting Newtonian behavior due to the low stability of the suspension.
This indicates that mechanical stirring of the XG solution with OSW-TPA was not sufficient to promote a chemical interaction between the waste material and the polymer, which would be necessary to enhance stability and improve rheological properties. A chemical reaction approach was applied to achieve a nanocomposite structure. It was also necessary to repeat these experiments at pH values > 9, where OSW-TPA was found to be more stable.
4. Conclusions
OSW particles were successfully modified with TPA, as confirmed by FTIR and zeta potential analysis, and their stability was increased under basic pH conditions. The fluids prepared with both pure and modified waste showed no strong physical interaction with XG through mechanical stirring for either type of particle, resulting in low viscosities and yield stress values. However, both fluids demonstrated shear-thinning behaviors. These results highlight the need to implement a chemical interaction between OSW-TPA and XG to enhance rheological properties and stability.
Author Contributions
Conceptualization, K.C.C.S.R.M. and C.J.D.; methodology, K.C.C.S.R.M. and C.J.D.; formal analysis, K.C.C.S.R.M.; data curation, K.C.C.S.R.M.; writing—original draft preparation, K.C.C.S.R.M.; writing—review and editing, K.C.C.S.R.M., C.J.D. and A.N.; supervision, C.J.D. and A.N.; project administration, C.J.D.; funding acquisition, C.J.D. and A.N. All authors have read and agreed to the published version of the manuscript.
Funding
CNPq—the National Council for Scientific and Technological Development, National Agency of Petroleum, Natural Gas and Biofuels (ANP), the Financier of Studies and Projects (FINEP—MCTI and FAPESP), through the PRH-ANP/MCTI 53.1 UFES, as well as CAPES, Grant number 001, and FAPES, Process number 2021-Q5DC4.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank the Graduate Program in Chemistry (PPGQUI) at UFES. Special thanks go to the laboratories Labpetro NCPQ/UFES, LabPol, LabReo, LMC, and LUCCAR at UFES.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khan, A.; Patidar, R.; Pappu, A. Marble waste characterization and reinforcement in low density polyethylene composites via injection moulding: Towards improved mechanical strength and thermal conductivity. Contr. Build. Mater. 2021, 269, 121229. [Google Scholar] [CrossRef]
- Gomes, V.; Babisk, M.; Vieira, C.; Sampaio, J.A.; Vidal, F.W.; Gadioli, M.C. Ornamental stone wastes as an alternative raw material for soda-lime glass manufacturing. Mater. Lett. 2020, 269, 127579. [Google Scholar] [CrossRef]
- Manca, P.P.; Orru, G.; Desogus, P. Recycling of sludge from ornamental stone processing as resource in civil constructions. Int. J. Mini. Reclamat. Environ. 2015, 29, 141–155. [Google Scholar] [CrossRef]
- Agustin, A.R.; Tamura, K. Surface modification of TiO2 nanoparticles with terephthalic acid in supercritical carbon dioxide. J. Supercrit. Fluids 2021, 174, 105245. [Google Scholar] [CrossRef]
- Mota, G.; Pereira, R. A comparison of the rheological behavior of xanthan gum and diutan gum in aqueous solutions. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 117. [Google Scholar] [CrossRef]
- Gudarzifar, H.; Sabbaghi, S.; Rezvani, A.; Saboori, R. Experimental investigation of rheological & filtration properties and thermal conductivity of water-based drilling fluid enhanced. Powder Technol. 2020, 368, 323–341. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).