1. Introduction
Colon cancer, one of the most prevalent human malignancies, represents a major public health concern in developed countries and ranks as the third leading cause of cancer-related mortality worldwide. A significant limitation of current chemotherapeutic regimens is their high cytotoxicity to normal cells. Consequently, the discovery of novel anticancer agents with selective activity and minimal side effects remains a critical priority [
1]. Moreover, up to 90% of colorectal cancer cases could be prevented through dietary modifications. In this context, edible berries have garnered increasing attention as potential sources of bioactive compounds with chemopreventive properties [
2].
The Lycium genus, which comprises 97 recognized species, is well known for its dual use in food and traditional medicine; of these, 31 species have documented pharmacological applications [
3]. Despite the broad interest in their bioactive properties, only two species—
Lycium barbarum and
Lycium ruthenicum—have been studied for their anticancer effects against human colon cancer. Carotenoid extracts and nanoemulsions from
Lycium barbarum were effective at inhibiting the growth of HT-29 colon cancer cells, with an IC
50 of 4.5 and 4.9 μg/mL, respectively [
4]; the LoVo cells, a typical human colorectal carcinoma cell line, were significantly inhibited by the mixture of the polysaccharides and anthocyanins from
Lycium ruthenicum, without imposing any cytotoxicity on normal immune cells [
5].
In Ukraine, Lycium chinense Mill. (L. chinense) (пoвiй китaйcький in Ukrainian) was introduced in 1998 into the dendrological park “Olexandria,” located in the northeastern sector of the Right-Bank properties region. The park is situated at an elevation of 80–106 m and experiences a temperate continental (Dfb) climate, with a mean annual temperature of 6.93 °C, ranging between 5.8 °C and 8.5 °C across different years. Data concerning the biological activity of Lycium fruits cultivated in temperate continental climates—particularly in Eastern Europe—remain exceedingly scarce.
The present study was therefore designed to obtain a polyphenolic extract from L. chinense, to evaluate its cytotoxicity against human colon cancer HT-29 cells and to characterize its antioxidant capacity.
2. Materials and Methods
2.1. Reagents and Chemicals
Unless otherwise indicated, all chemicals and solvents were purchased from Merck (Madrid, Spain). Aluminum chloride and sodium carbonate were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). Water was purified using a Milli-Q system (Millipore, Burlington, MA, USA). All the chemicals used, including the solvents, were of analytical grade.
2.2. Plant Material
Fruits were manually harvested at maturity from three plants in the dendrological park “Olexandria”. The voucher number of the collected species is 09113/2024. Washed fruits were dried at 40 °C for 24 h and stored at 4 °C for further use.
2.3. Extraction and Determination of Total Phenolic and Flavonoid Contents
The berries were defatted with hexane, and then we extracted methanol/water (60:40,
v/
v) using a Soxhlet apparatus. After experimenting with different solvent ratios, the ratio between both solvents was selected because it yields the highest amount of phenolics. After centrifuging at 3000×
g for 15 min [
6], the supernatants were collected and combined, and the solvent was evaporated under vacuum at 60 °C to dryness. The residue was used for the determination of total phenolic content (TPC) and total flavonoid content. The determination of the TPC was conducted following the Folin–Ciocalteu procedure [
7]. Absorbance values at 725 nm were then converted to Gallic Acid Equivalents (GAEs) using gallic acid as the reference compound. The total flavonoid content was determined using the aluminum chloride method, with the absorbance of the samples being measured at 420 nm. The results were expressed as rutin equivalents in milligrams per gram dry weight of plant material [
8].
2.4. Antioxidant Activity
2.4.1. DPPH Method
The DPPH assay was performed according to the method described [
9]. Ascorbic acid was used as positive antioxidant control.
2.4.2. β-Carotene Bleaching Method
Antioxidant activity was determined by measuring inhibition of
β-carotene bleaching as described [
10] with modifications. The reagent consisted of 1 mL
β-carotene (2 mg/mL in chloroform), 20 mg of linoleic acid and 100 mg of Tween 80, which were vigorously mixed by vortexing and flushed with nitrogen to remove chloroform before addition of oxygen-sparged distilled water (100 mL). Samples (100 µg/mL; 200 µL) were added to 800 µL of the
β-carotene reagent and incubated in the dark at 54 °C for 60 min before determination of absorbance at 450 nm. Butylated hydroxytoluene (BHT) was used as reference antioxidant.
2.5. Cell Assays on Cancer and Normal Cell Lines
The HT-29: Human Colorectal Adenocarcinoma Cell Line (ATCC HTB-38American Type Culture Collection) and the CCD-18Co colonic human myofibroblasts cells line (CRL-1459 ™) were used to check antiproliferative activities. HT-29 cell line is a well-established model used to study the biology of human colon cancers but also in studies focused on food bioavailability and digestion due to epithelial morphology [
2]. Cultures were supplied by the Technical Instrumentation Service of University of Granada (Granada, Spain). Cells were grown at 37 °C and 5% CO
2 humidified atmosphere in medium RPMI-1640 supplemented with 5% fetal bovine serum, 2 mM
L-glutamine, 1 mM sodium pyruvate, 0.125 mg/mL amphotericin and 100 mg/mL penicillin–streptomycin.
All cultures were plated in 25 cm
2 plastic tissue culture flasks (Sarstedt, Newton, NC, USA). All culture media and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell culture and cell assays, that is, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) test, were accomplished as previously described [
11]. Values presented are mean ± standard error of the mean. The selectivity index (SI) was calculated as GI
50 of the extract against the CCD-18 normal cell line/GI
50 of the same extract against the HT-29 cancer cell line [
12].
2.6. Statistical Analysis
All analyses were performed in triplicate. All data were expressed as the average ± SD. The statistical significance was calculated using Student’s t-tests and a one-way analysis of variance (ANOVA) and expressed as the average SD. Differences among mean values were tested by Duncan’s test at p < 0.05 and for antiproliferative activity at p < 0.05 and p < 0.01. Statistical analyses were performed using Statgraphics Centurion XVIII version. 18.1.12 (Warrenton, VA, USA).
3. Results and Discussion
3.1. Total Phenolics and Flavonoids Content
The hydroalcoholic extraction of goji berries, carried out under the previously described conditions, resulted in a yield of 16.9% dry weight. The TPC was 11.3 mg GAE/g dry weight, while the total flavonoid content was 7.3 mg rutin/g dry weight. These values are comparable to those reported for commercial samples obtained from the Brazil Municipal Market [
13]. The TPC was higher than the TPC in the small cranberry
Vaccinium oxycoccos (3.72 GAE/g DW) and was comparable with the TPC in other well-known edible berries, such as
Vaccinium myrtillus (2.20–37.15 GAE/g DW) and
Hippophae rhamnoides (9.3 GAE/g DW) [
14]. The extract exhibited an antioxidant activity, with a DPPH radical scavenging IC
50 value of 125.9 ± 12.6 µg/mL and a lipid peroxidation inhibition of 23.5 ± 2.1% in the
β-carotene bleaching assay. However, its antioxidant capacity was considerably lower when compared to the positive controls: the ascorbic acid (IC
50 = 4.2 ± 1.0 µg/mL) in the DPPH assay and BHT (78.6 ± 0.8% inhibition) in the
β-carotene assay.
3.2. Anticancer Activity Against HT-29 Colon Cancer Cells
The
L. chinense extract did not inhibit the growth of HT-29 after the 48 h incubation. After 72 h of treatment, the MTT assay demonstrated a concentration- and time-dependent inhibitory effect on HT-29 cells (
Figure 1).
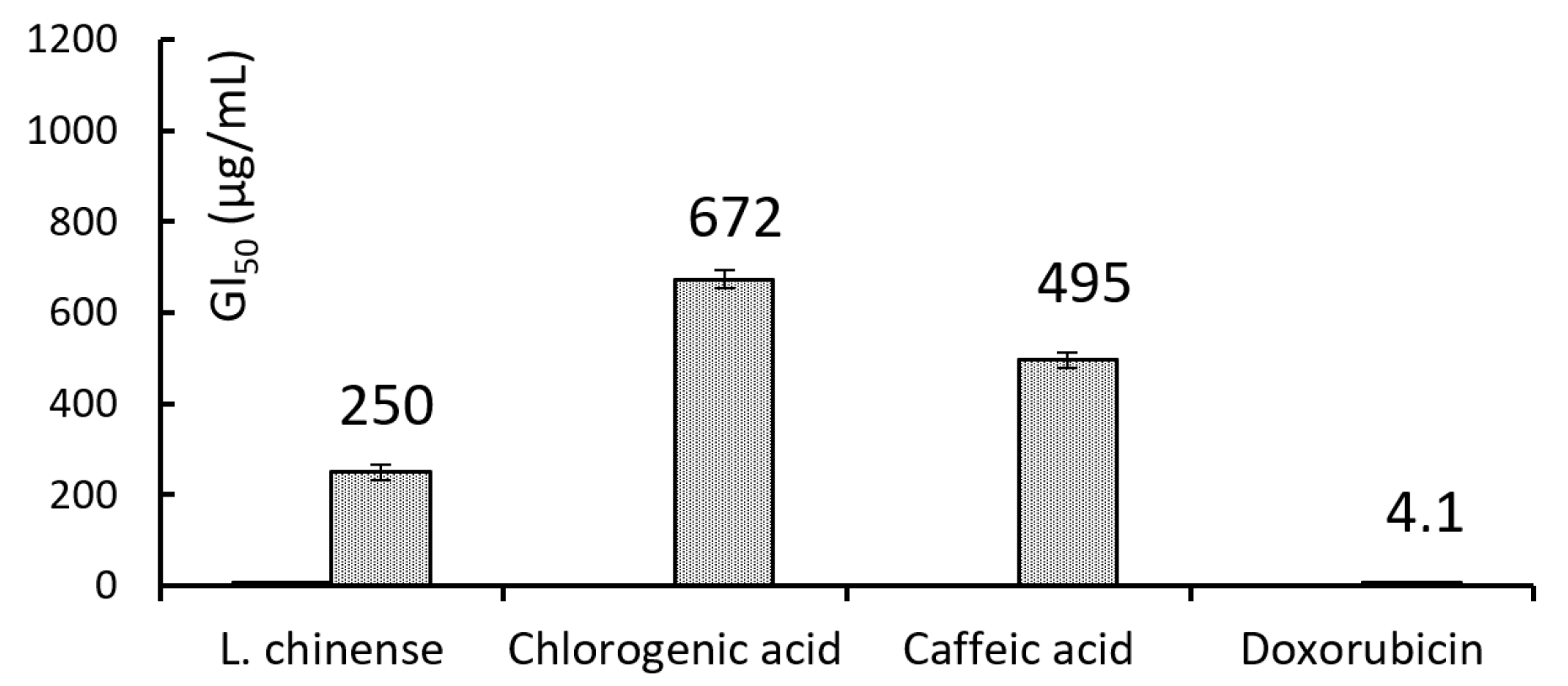
The GI
50 values of the extract, doxorubicin (control) and selected phenolic compounds are shown in
Figure 2.
It is important to consider that polysaccharide-rich fractions from
Lycium species have been previously reported as highly bioactive [
15]. Given that the water/methanol extracts used in this study may contain certain amounts of polysaccharides, a synergistic interaction between phenolic compounds and seed-derived polysaccharides may contribute to the observed anticancer effects—particularly considering that HT-29 cells are known to exhibit a limited sensitivity to phenolic compounds alone [
10]. A similar synergistic effect has previously been reported in
L. ruthenicum, where a combination of polysaccharides and anthocyanins demonstrated an inhibitory activity against human colorectal carcinoma LoVo cells [
5]. Additionally, the SI was calculated to compare the extract’s cytotoxicity toward HT-29 cancer cells and normal cells CCD-18. An SI value greater than two indicates a high selectivity toward cancer cells, whereas values below two suggest a general cytotoxicity to both normal and cancer cells. Such a value for any research on herbal drugs and/or isolated compounds is critical for determining whether further research can be continued [
16]. The SI at 72 h was 10, from which it can be concluded that the
Lycium extract has a high selectivity against HT-29 human colorectal cancer cells. This means that the extract evaluated here constitutes a promising candidate for the possible obtainment of active compounds against colorectal cancer.
4. Conclusions
This study provides the first evidence of the anticancer potential of L. chinense fruits cultivated in Ukraine. The phenolic extract exhibited a selective cytotoxicity against HT-29 colorectal cancer cells, highlighting its promise as a source of bioactive compounds for nutraceutical or pharmaceutical applications. Future research should prioritize elucidating the molecular mechanisms underlying its antiproliferative effects, conducting a detailed characterization of phenolic constituents, developing regionally adapted cultivars with optimized bioactivity and promoting the sustainable valorization of processing by-products. Given the influence of environmental factors on chemical compositions, long-term studies are essential to ensure consistency and efficacy in the context of human health.
Author Contributions
Conceptualization, S.L. and J.L.G.-G.; methodology, S.L. and J.L.G.-G.; software, S.L. and N.B.; validation, S.L., N.B., V.S. and J.L.G.-G.; formal analysis, N.B. and V.S.; investigation, S.L.; resources, S.L., N.B., V.S. and J.L.G.-G.; data curation, S.L., N.B., V.S. and J.L.G.-G.; writing—original draft preparation, S.L.; writing—review and editing, S.L., N.B., V.S. and J.L.G.-G.; visualization, S.L., N.B., V.S. and J.L.G.-G.; supervision, J.L.G.-G. and S.L.; project administration, J.L.G.-G. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TPC | Total phenolic compounds |
| TFC | Total flavonoid content |
| DPPH | 2’-diphenyl-1-picrylhydrazyl radical |
| BHT | Butylated hydroxytoluene |
References
- Lei, M.; Guanying, B.X.; Xinyu, T.; Cheng, Z.; Wen, Z.; Jie, W.; Hong, C. Anti-cancer potential of polysaccharide extracted from hawthorn (Crataegus.) on human colon cancer cell line HCT116 via cell cycle arrest and apoptosis. J. Funct. Foods 2020, 64, 103677. [Google Scholar] [CrossRef]
- Kiernozek, E.; Maslak, P.; Kozlowska, E.; Jarzyna, I.; Średnicka-Tober, D.; Hallmann, E.; Kazimierczak, R.; Drela, N.; Rembiałkowska, E. Biological Activity of Extracts from Differently Produced Blueberry Fruits in Inhibiting Proliferation and Inducing Apoptosis of HT-29 Cells. Foods 2022, 11, 3011. [Google Scholar] [CrossRef] [PubMed]
- Baimakhanova, B.; Sadanov, A.; Bogoyavlenskiy, A.; Berezin, V.; Trenozhnikova, L.; Baimakhanova, G.; Ibraimov, A.; Serikbayeva, E. Exploring phytochemicals and their pharmacological applications from ethnomedicinal plants: A focus on Lycium barbarum, Solanacea. Heliyon 2025, 11, e41782. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.J.; Huang, R.F.; Kao, T.H.; Inbaraj, B.S.; Chen, B.H. Preparation of carotenoid extracts and nanoemulsions from Lycium barbarum L. and their effects on growth of HT-29 colon cancer cells. Nanotechnology 2017, 28, 135103. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, X.; Xu, K.; Yang, X.; Wang, Q.; Liu, C.; Wang, X.; Guo, X.; Sun, J.; Li, L.; et al. Synergistic antitumor effects of polysaccharides and anthocyanins from Lycium ruthenicum Murr. on human colorectal carcinoma LoVo cells and the molecular mechanism. Food Sci. Nutr. 2022, 18, 2956–2968. [Google Scholar] [CrossRef] [PubMed]
- Yossa, N.; Guo, B.; Zhang, T.; Wang, L.; Ji, Q.; Xia, H.; Sun, G. Comparative Metabolic Profiling of Lycium Fruits (Lycium barbarum and Lycium chinense) from Different Areas in China and from Nepal. J. Food Qual. 2019, 2019, 4396027. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: London, UK, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Mocan, A.; Cairone, F.; Locatelli, M.; Cacciagrano, F.; Carradori, S.; Vodnar, D.C.; Crișan, G.; Simonetti, G.; Cesa, S. Polyphenols from Lycium barbarum (Goji) Fruit European Cultivars at Different Maturation Steps: Extraction, HPLC-DAD Analyses, and Biological Evaluation. Antioxidants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Benchennouf, A.; Grigorakis, S.; Loupassaki, S.; Kokkalou, E. Phytochemical analysis and antioxidant activity of Lycium barbarum (Goji) cultivated in Greece. Pharm. Biol. 2017, 55, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Castilla, J.; Hernández-Álvarez, A.J.; Jiménez-Martínez, C.; Jacinto-Hernández, C.; Alaiz, M.; Girón-Calle, J.; Vioque, J.; Dávila-Ortiz, G. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem. 2012, 135, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Lyashenko, S.; López-Ruiz, R.; García-Cervantes, A.M.; Rodríguez-García, I.; Yunusova, S.; Guil-Guerrero, J.L. Phenolic Profiles and Antitumor Activity against Colorectal Cancer Cells of Seeds from Selected Ribes Taxa. Appl. Sci. 2024, 14, 2428. [Google Scholar] [CrossRef]
- Vichitsakul, K.; Laowichuwakonnukul, K.; Soontornworajit, B.; Poomipark, N.; Itharat, A.; Rotkrua, P. Anti-proliferation and induction of mitochondria-mediated apoptosis by Garcinia hanburyi resin in colorectal cancer cells. Heliyon 2023, 22, e16411. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.; Reis, A.S.; Silva, L.D.; Lima, V.A.; Oldoni, T.L.C.; Pereira, C.; Carpes, S.T. Optimization of phenolic compounds extraction with antioxidant activity from açaí, blueberry and goji berry using response surface methodology. Emir. J. Food Agric. 2018, 30, 180–189. [Google Scholar]
- Hidalgo, G.-I.; Almajano, M.P. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.R.; Vestuto, V.; Amodio, G.; Manfra, M.; Pepe, G.; Campiglia, P. Antitumor Mechanisms of Lycium barbarum Fruit: An Overview of In Vitro and In Vivo Potential. Life 2024, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).