A Three-Step Approach to Estimation of Reduction Potentials of Natural Mixtures of Antioxidants Based on DPPH Test; Illustration for Catechins and Cocoa †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
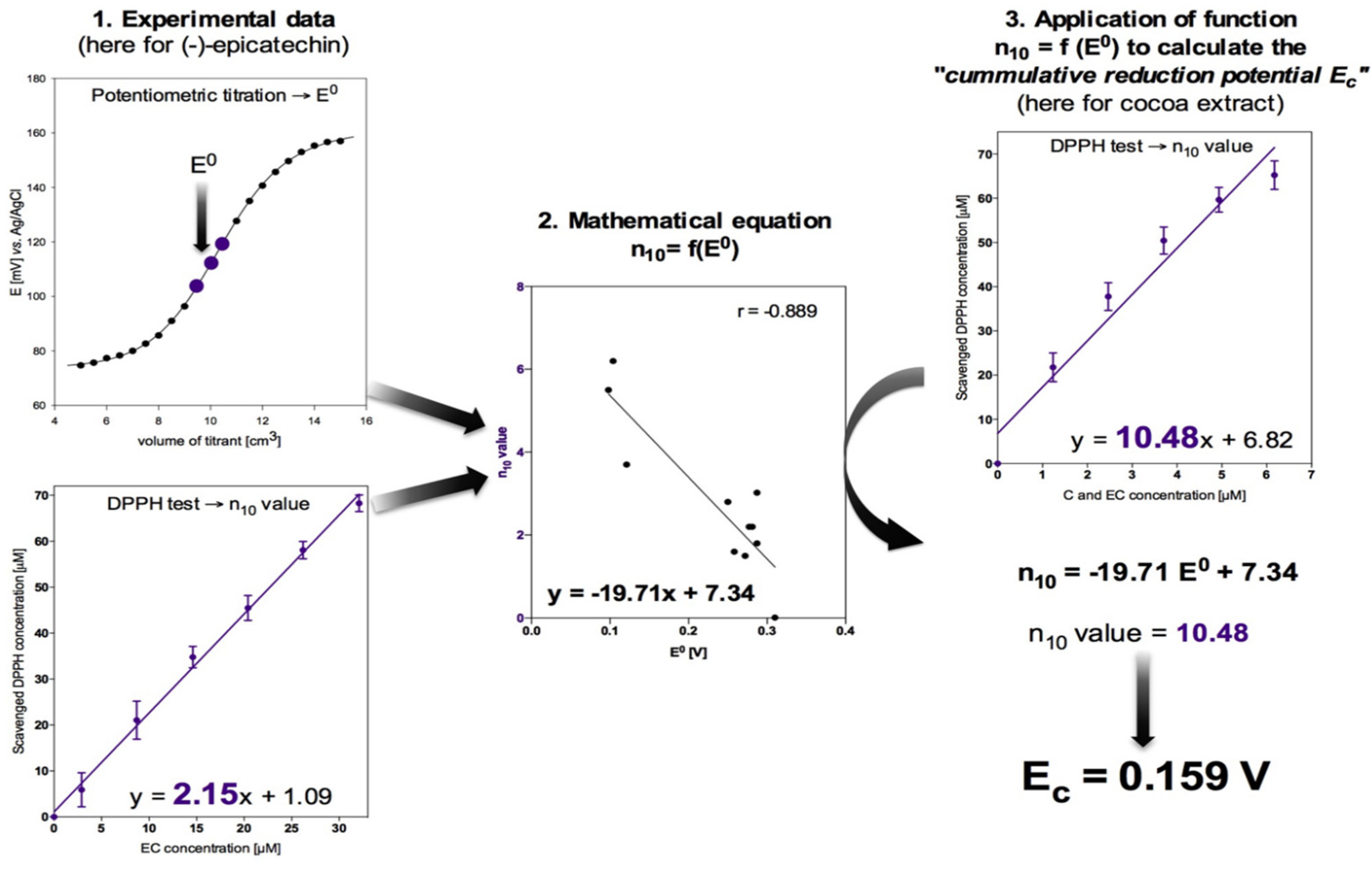
2.2. Standard Reduction Potential by Potentiometric Titration
2.3. Antioxidant Activity by Spectrophotometic Method
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Baranowska, M.; Suliborska, K.; Chrzanowski, W.; Kusznierewicz, B.; Namieśnik, J.; Bartoszek, A. The relationship between standard reduction potentials of catechins and biological activities involved in redox control. Redox Biol. 2018, 17, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Goupy, P.; Dufour, C.; Loonis, M.; Dangles, O. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J. Agric. Food Chem 2003, 51, 615–622. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusznierewicz, B.; Baranowska, M.; Suliborska, K.; Chrzanowski, W.; Bartoszek, A. A Three-Step Approach to Estimation of Reduction Potentials of Natural Mixtures of Antioxidants Based on DPPH Test; Illustration for Catechins and Cocoa. Proceedings 2019, 11, 4. https://doi.org/10.3390/proceedings2019011004
Kusznierewicz B, Baranowska M, Suliborska K, Chrzanowski W, Bartoszek A. A Three-Step Approach to Estimation of Reduction Potentials of Natural Mixtures of Antioxidants Based on DPPH Test; Illustration for Catechins and Cocoa. Proceedings. 2019; 11(1):4. https://doi.org/10.3390/proceedings2019011004
Chicago/Turabian StyleKusznierewicz, Barbara, Monika Baranowska, Klaudia Suliborska, Wojciech Chrzanowski, and Agnieszka Bartoszek. 2019. "A Three-Step Approach to Estimation of Reduction Potentials of Natural Mixtures of Antioxidants Based on DPPH Test; Illustration for Catechins and Cocoa" Proceedings 11, no. 1: 4. https://doi.org/10.3390/proceedings2019011004
APA StyleKusznierewicz, B., Baranowska, M., Suliborska, K., Chrzanowski, W., & Bartoszek, A. (2019). A Three-Step Approach to Estimation of Reduction Potentials of Natural Mixtures of Antioxidants Based on DPPH Test; Illustration for Catechins and Cocoa. Proceedings, 11(1), 4. https://doi.org/10.3390/proceedings2019011004



