Comprehensive Update on European Union Labeling Standards for Coffee and Its By-Products †
Abstract
1. Introduction
| Coffee By-Product | Novel Food Status a | EFSA Opinion | EC Implementing Regulation |
|---|---|---|---|
| Flowers (blossoms) | Probably novel, currently not approved. Some anecdotal evidence for traditional food uses in third country. Needs approval procedure. | - | - |
| Stems, twigs, and wood | Non-food material, contamination up to certain levels typically tolerated in the trade of green coffee. | - | - |
| Leaves | Novel, authorization granted for coffee leaves (herbal infusion and beverage uses) based on notification as traditional food from third country [15]. | [7] | [6,10] |
| Coffee cherry materials (husks, cascara, dried or fresh coffee cherries, and coffee pulp or mucilage) | Novel, authorization granted for cascara and cherry pulp (beverage uses) based on notifications as traditional food from third country [16,17]. | [8,9] | [4] |
| Parchment | Probably novel, currently not approved. No application pending. Needs approval procedure. | - | - |
| Green unroasted beans (seeds) | Probably not novel [18]. The current version of the EU novel food status catalogue eliminated the specific entry for green unroasted beans (for previous 2020 version, see Figure 1 in Klingel et al. [1]). It can be assumed that the new entry for seeds of Coffea spp. as not novel also applies to the green unroasted beans as roasting is not specified in the entry. The classification as not novel would then also apply to the non-selective water extraction made of green unroasted seeds. Selective extracts are probably novel. | - | - |
| Silver skin | Novel [19]. Probably not a traditional food from third country. Needs full approval procedure. | - | - |
| Coffee grounds | Not novel (spent coffee grounds, defatted spent coffee grounds, and defatted unused coffee grounds) [20]. | - | - |
| Coffee grounds oil extract | Novel [21]. Probably not a traditional food from third country. Needs full approval procedure. | - | - |

2. Regulations for Coffee Leaves
3. Regulations for Coffee Cherry (Cascara)
4. General Labeling Requirements of Foods Based on Coffee and Coffee By-Products in the EU
- the name of the food (for by-products according to the designation in the Novel Food approvals [23])
- the net quantity of the food
- the date of minimum durability/lot labeling (in accordance with the Directive 2011/91/EU [24]
- instructions for use if necessary (such as the preparation of a coffee leaf tea with the required pasteurization or infusion)
- the name or business name and the address of a food business operator
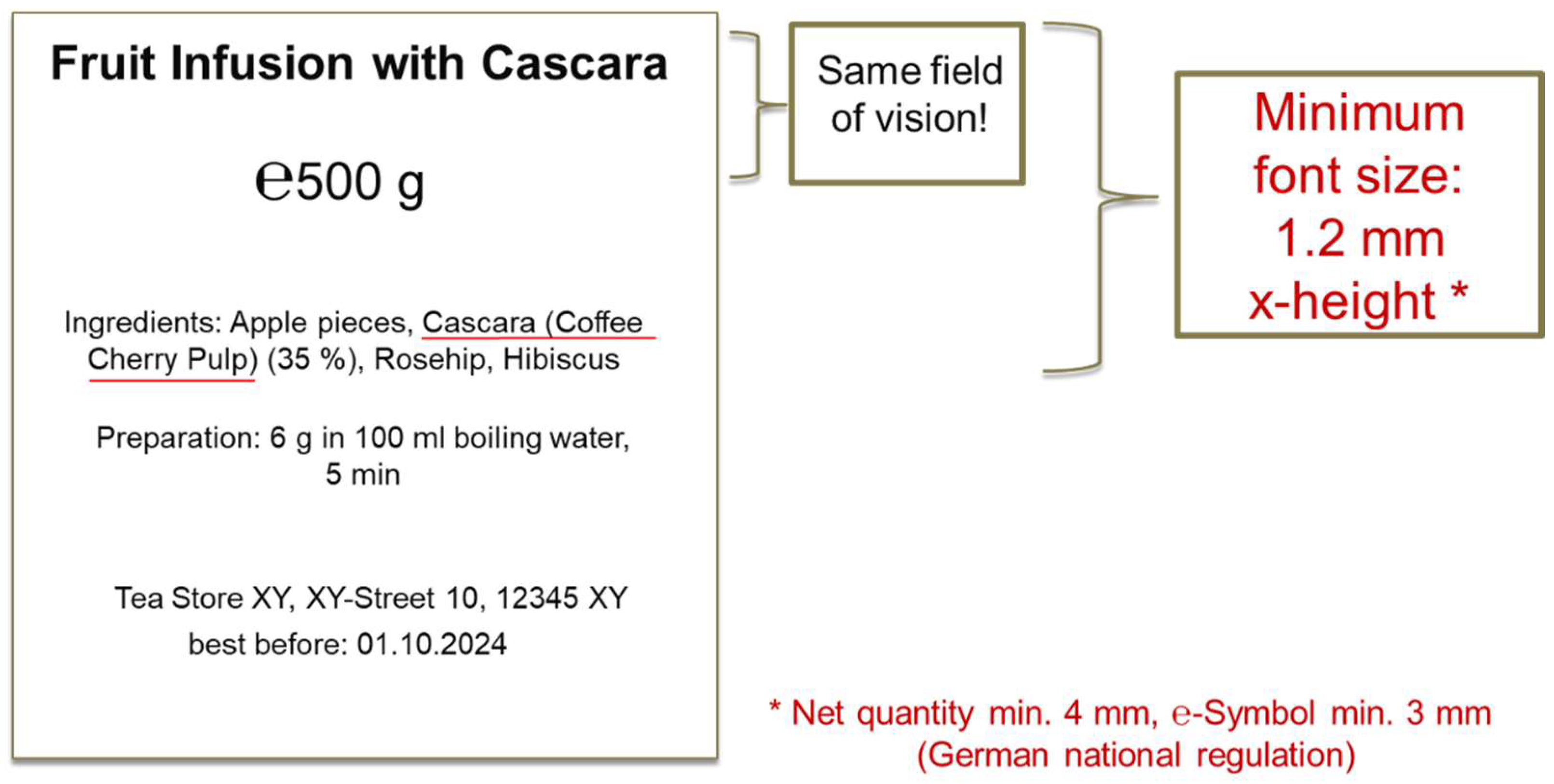
5. Some Specific Details for Mandatory Labeling Elements
5.1. Name of the Food
5.2. Net Quantity
5.3. Minimum Durability Date/Lot Labeling
5.4. Instructions for Use
5.5. Name or Business Name and Address of a Food Business Operator
6. Challenges in Labeling of Foods Containing Coffee By-Products
7. Voluntary Labeling Claims and Online Commerce
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klingel, T.; Kremer, J.I.; Gottstein, V.; De Rezende, T.R.; Schwarz, S.; Lachenmeier, D.W. A Review of Coffee By-Products Including Leaf, Flower, Cherry, Husk, Silver Skin, and Spent Grounds as Novel Foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Schwarz, S.; Rieke-Zapp, J.; Cantergiani, E.; Rawel, H.; Martín-Cabrejas, M.A.; Martuscelli, M.; Gottstein, V.; Angeloni, S. Coffee By-Products as Sustainable Novel Foods: Report of the 2nd International Electronic Conference on Foods—“Future Foods and Food Technologies for a Sustainable World”. Foods 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Rajcic de Rezende, T.; Schwarz, S. An Update on Sustainable Valorization of Coffee By-Products as Novel Foods within the European Union. Biol. Life Sci. Forum 2021, 6, 37. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (eu) 2022/47 of 13 January 2022 Authorising the Placing on the Market of Coffea arabica L. and/or Coffea canephora Pierre Ex A. Froehner Dried Cherry Pulp and Its Infusion as a Traditional Food from a Third Country Under Regulation (eu) 2015/2283 of the European Parliament and of the Council and Amending Commission Implementing Regulation (eu) 2017/2470. Off. J. Europ. Union 2022, L9, 29–32. [Google Scholar]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Dried Coffee Husk (Cascara) from Coffea arabica L. as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07085. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2020/917 of 1 July 2020 Authorising the Placing on the Market of Infusion from Coffee Leaves of Coffea arabica L. and/or Coffea canephora Pierre Ex A. Froehner as a Traditional Food from a Third Country Under Regulation (EU) 2015/2283 of the European Parliament and of the Council and Amending Implementing Regulation (EU) 2017/2470. Off. J. Europ. Union 2020, L209, 10–13. [Google Scholar]
- European Food Safety Authority (EFSA). Technical Report on the Notification of Infusion from Coffee Leaves (Coffea arabica L. and/or Coffea canephora Pierre Ex a. Froehner) as a Traditional Food from a Third Country Pursuant to Article 14 of Regulation (EU) 2015/2283. EFSA Supp. Publ. 2020, 17, 1783E. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Technical Report on the Notification of Cherry Pulp from Coffea arabica L. and Coffea canephora Pierre Ex A. Froehner as a Traditional Food from a Third Country Following Article 14 of Regulation (EU) 2015/2283. EFSA Support. Publ. 2021, 18, 6657E. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Technical Report on the Notification of Dried Cherry Pulp from Coffea arabica L. and Coffea canephora Pierre Ex A. Froehner as a Traditional Food from a Third Country Pursuant to Article 14 of Regulation (EU) 2015/2283. EFSA Supp. Publ. 2021, 18, 6808E. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2023/931 of 8 May 2023 Amending Implementing Regulation (EU) 2017/2470 as Regards the Conditions of Use of the Traditional Food from a Third Country Infusion from Coffee Leaves of Coffea arabica L. and/or Coffea canephora Pierre Ex A. Froehner. Off. J. Europ. Union 2023, L124, 1–3. [Google Scholar]
- Panama Varietals GmbH. Coffee Husk (Cascara)—The Dried Husk of the Coffee Fruit or Coffee Cherry. Available online: https://ec.europa.eu/food/system/files/2020-03/novel-food_sum_ongoing-app_2018-0192.pdf (accessed on 12 August 2021).
- European Commission. Commission Implementing Decision of 23 November 2022 Terminating the Procedure for Authorising the Placing on the Market of Dried Coffee Cherry Husk (cascara) from Coffea arabica L. as a Novel Food Without Updating the Union List of Novel Foods (c(2022)8307). Available online: https://food.ec.europa.eu/safety/novel-food/decisions-terminating-procedure_en (accessed on 16 April 2024).
- European Parliament and Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Europ. Union 2021, L327, 1–22. [Google Scholar]
- European Parliament and Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004; European Parliament and Council of the European Union: Brussels, Belgium, 2011; Volume L304, pp. 18–63. [Google Scholar]
- AM Breweries IVS. Herbal Infusion Made from Coffee Leaves. Available online: https://ec.europa.eu/food/system/files/2019-11/novel-food_sum_ongoing-not_2018-0740.pdf (accessed on 12 August 2021).
- Societé de Produits Nestle SA. Coffee Cherry Pulp. Available online: https://ec.europa.eu/food/system/files/2021-02/novel-food_sum_ongoing-not_2020-1762.pdf (accessed on 12 August 2021).
- Luigi Lavazza SpA. Dried Coffee Cherry of Coffea sp. (Cascara). Available online: https://ec.europa.eu/food/system/files/2021-04/novel-food_sum_ongoing-not_2019-1026.pdf (accessed on 12 August 2021).
- European Union Novel Food Catalogue. Coffea sp. Available online: https://ec.europa.eu/food/food-feed-portal/screen/novel-food-catalogue/search (accessed on 16 April 2024).
- Recipient Member State: Germany. Roasted Silver Skin of Coffee Beans (Testa of Coffea sp.). Consultation on the Determination of the Status of a Novel Food under Article 4 (2) of Regulation (EU) 2015/2283. Available online: https://food.ec.europa.eu/document/download/03b97969-b53f-4745-a66f-fbf1eaabe167_en?filename=novel-food_consult-status_2022-4778355.pdf (accessed on 16 April 2024).
- Danish Veterinary and Food Administration (DVFA). Consultation Request to Determine the Status of Spent Coffee Grounds, Defatted Spent Coffee Grounds and Defatted Unused Coffee Grounds Pursuant to Article 4(2) of Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods. Available online: https://ec.europa.eu/food/system/files/2021-03/novel-food_consult-status_coffee-grounds.pdf (accessed on 8 September 2021).
- Czech Republic—Ministry of Agriculture—Food Safety Department. Consultation Request to Determine the Novel Food Status of Coffee (Coffea arabica) Grounds Oil Extract. Available online: https://food.ec.europa.eu/document/download/98c97f9a-5501-4e2a-bb99-4ce55f217597_en?filename=novel-food_consult-status_coffee-grounds-oil-extract.pdf (accessed on 12 May 2024).
- Steger, M.C.; Rigling, M.; Blumenthal, P.; Segatz, V.; Quintanilla-Belucci, A.; Beisel, J.M.; Rieke-Zapp, J.; Schwarz, S.; Lachenmeier, D.W.; Zhang, Y. Coffee Leaf Tea from El Salvador: On-Site Production Considering Influences of Processing on Chemical Composition. Foods 2022, 11, 2553. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. Off. J. Europ. Union 2024, L351, 72–201. [Google Scholar]
- European Parliament and Council of the European Union. Directive 2011/91/EU of the European Parliament and of the Council of 13 December 2011 on Indications or Marks Identifying the Lot to Which a Foodstuff Belongs (Codification). Off. J. Europ. Union 2011, L334, 1–5. [Google Scholar]
- Verordnung über Kaffee, Kaffee- und Zichorien-Extrakte. Available online: https://www.gesetze-im-internet.de/kaffeev_2001/BJNR310700001.html (accessed on 12 May 2024).
- Verordnung über Fertigpackungen und andere Verkaufseinheiten (Fertigpackungsverordnung—FPackV). Available online: https://www.gesetze-im-internet.de/fpackv/BJNR250410020.html (accessed on 12 May 2024).
- Monakhova, Y.B.; Ruge, W.; Kuballa, T.; Ilse, M.; Winkelmann, O.; Diehl, B.; Thomas, F.; Lachenmeier, D.W. Rapid Approach to Identify the Presence of Arabica and Robusta Species in Coffee Using 1H NMR Spectroscopy. Food Chem. 2015, 182, 178–184. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council of the European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Europ. Union 2006, L404, 9–25. [Google Scholar]


| Authorized Novel Food | Infusion from Coffee Leaves of Coffea arabica L. and/or Coffea canephora Pierre ex A. Froehner (Traditional Food from a Third Country) |
|---|---|
| Specified food category | Infusion from coffee leaves of Coffea arabica L. and/or Coffea canephora Pierre ex A. Froehner placed on the market as such; Flavored and unflavored non-alcoholic ready-to-drink beverage; Coffee, coffee and chicory extracts, instant coffee, tea, herbal- and fruit-infusions, coffee substitutes, coffee mixes and instant mixes for beverages (and their flavored counterparts). |
| Additional specific labeling requirements | The designation of the novel food on the labeling of the foodstuffs containing it shall be ‘Infusion from coffee leaves’ or ‘Dried infusion from coffee leaves’, depending on the form to be marketed. |
| Description/definition | The traditional food consists of an infusion of leaves from Coffea arabica L. and/or Coffea canephora Pierre ex A. Froehner (family: Rubiaceae). |
| The traditional food is prepared by mixing a maximum of 20 g of dried leaves from Coffea arabica L. and/or Coffea canephora Pierre ex A. Froehner with 1 L of hot water. Leaves are removed and the infusion is then subjected to pasteurization (at least 71 °C for 15 s). | |
| Composition | Visual: brown green liquid |
| Odor and taste: characteristic | |
| Chlorogenic acid (5-CQA): <100 mg/L | |
| Caffeine: <80 mg/L | |
| Epigallocatechin gallate (EGCG): <700 mg/L | |
| Microbiological criteria | Total plate count: <500 CFU/g |
| Total yeast and mold count: <100 CFU/g | |
| Total coliforms: <100 CFU/g | |
| Escherichia coli: absence in 1 g | |
| Salmonella: absence in 25 g | |
| Heavy metals | Lead (Pb): <3.0 mg/L |
| Arsenic (As): <2.0 mg/L | |
| Cadmium (Cd): <1.0 mg/L |
| Authorized Novel Food | Coffea arabica L. and/or Coffea canephora Pierre ex A. Froehner Dried Cherry Pulp and Its Infusion (Traditional Food from a Third Country) |
|---|---|
| Specified food category | Coffee cherry pulp from Coffea arabica L. and/or Coffea canephora Pierre ex A. Froehner for the preparation of infusions; Coffee, coffee and chicory extracts, instant coffee, tea, herbal- and fruit-infusions, coffee substitutes, coffee mixes and instant mixes for hot beverages (and their flavored counterparts); Flavored and unflavored non-alcoholic ready-to-drink beverages. |
| Additional specific labeling requirements | The designation of the novel food on the labeling of the foodstuffs containing it shall be “coffee cherry pulp” and/or “cascara (coffee cherry pulp)”, and/or “coffee cherry pulp infusion” and/or “coffee cherry pulp dried infusion”. If the product containing the novel food contains more than 150 mg/L of caffeine (as such or after reconstitution), it shall be labeled with the following indication: “High caffeine content. Not recommended for children or pregnant or breast-feeding women” in the same field of vision as the name of the food, followed by the caffeine content expressed in mg per 100 mL. Typical infusion preparations are prepared with up to 6 g of coffee cherry pulp per 100 mL of hot water (>75 °C). For the coffee cherry pulp placed on the market as such for the preparation of infusions, instructions shall be given to the consumer on the preparation. |
| Description/definition | The traditional food consists of the dried unroasted coffee cherry pulp of Coffea arabica L. and/or Coffea canephora Pierre ex A. Froehner (genus: Coffea family: Rubiaceae) and its infusion. The infusion can be used as such or concentrated or dried. Ripe coffee cherries are collected, and then the coffee beans are mechanically removed, prior or after a drying process, leaving the dried coffee cherry pulp, which can be milled to a powder. The separated coffee cherry pulp is also known as “cascara”, from the Spanish “cáscara”, meaning “husk”. Typically, the infusion is prepared by mixing up to 6 g of cascara pulp or husk in 100 mL of hot water (>75 °C) for a few minutes and then pouring through a strainer, or using corresponding amounts in dried or instant infusions. |
| Composition | Water: <18% |
| Water activity (aw): ≤0.65 | |
| Ash: <10.4% DM | |
| Protein: <15% DM | |
| Fat: <5% DM | |
| Carbohydrates: <85% DM | |
| Microbiological criteria | Aerobic Plate Count: <104 CFU/g |
| Total yeasts and molds: <100 CFU/g | |
| Enterobacteriaceae: <50 CFU/g | |
| Salmonella: Absence in 25 g | |
| Bacillus cereus: <100 CFU/g | |
| Mycotoxins | Ochratoxin A: <5.0 μg/kg |
| Aflatoxin B1: <2.0 μg/kg | |
| Aflatoxin B1. B2. G1. G2 (as sum): <4.0 μg/kg | |
| Heavy metals | Cadmium (Cd): <0.05 mg/kg |
| Lead (Pb): <1.0 mg/kg | |
| Copper: ≤50 mg/kg | |
| Mercury: ≤0.02 mg/kg | |
| Arsenic: ≤0.2 mg/kg | |
| Impurities | Benzo(a)pyrene: <10.0 μg/kg |
| Sum of benzo(a)pyrene, benz(a)anthracene, benzo(b)fluoranthene and chrysene: <50.0 μg/kg | |
| Pesticides | Pesticide levels in the traditional food shall comply with levels set by Regulation (EC) No 396/2005 for “0639000” for “Herbal infusions from any other parts of the plant”. |
| Product Group | Legal Designation According to Commission Implementing Regulation (EU) 2017/2470 [23] | Comments about Issues in Interpreting the Commission Implementing Regulation (EU) 2017/2470 [23] | Teleological Suggestion for Labeling a |
|---|---|---|---|
| Coffee leaves as such | Dried infusion from coffee leaves | The legal designation makes no sense for dried leaves as such. In the strictest legal interpretation, only the “infusion” would be able to be imported into the EU. There may be a translation error, i.e., in some languages “infusion” may also refer to the solid herbal plant material. Infusion may have been used, as the word “tea” is often believed as only appropriate for infusions of Camelia sinensis. For example, the German word “Kräutertee” is a correct designation also for the herbs as such. Hence the term “infusion of coffee leaves” may have been inappropriately translated to “Aufguss aus Kaffeeblättern”, while the translation “Kaffeeblatt-Tee” may have been more appropriate, which would holistically include both solid tea leaves as well as the beverage prepared in the form of an aqueous infusion. | Front label: Coffee leaf tea Back label: Coffee leaves for preparation of an infusion (same field of vision as net quantity) |
| Coffee leaves as an ingredient in flavored and unflavored non-alcoholic ready-to-drink beverages | “Infusion from coffee leaves” in the list of ingredients | - | - |
| Coffee leaves as an ingredient in instant mixes for beverages | “Dried infusion from coffee leaves” in the list of ingredients | - | - |
| Cascara from dry processing (dried coffee cherry) | “Coffee cherry pulp” and/or “cascara (coffee cherry pulp)” | The legal designation “pulp” is not completely correct for cascara from dry processing (dried husks containing the peel, pulp, and parchment) | Front label: Cascara or Coffee cherry Back label: “Coffee cherry pulp” and/or “Cascara (coffee cherry pulp)”, and/or “Coffee cherry pulp infusion” and/or “Coffee cherry pulp dried infusion” (same field of vision as net quantity) |
| Cascara from wet processing (dried cherry pulp) | |||
| Cascara as an ingredient in flavored and unflavored non-alcoholic ready-to-drink beverages | “Coffee cherry pulp infusion” and/or “coffee cherry pulp dried infusion” in the list of ingredients | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kull, A.-K.; Lachenmeier, D.W. Comprehensive Update on European Union Labeling Standards for Coffee and Its By-Products. Proceedings 2024, 109, 19. https://doi.org/10.3390/ICC2024-17350
Kull A-K, Lachenmeier DW. Comprehensive Update on European Union Labeling Standards for Coffee and Its By-Products. Proceedings. 2024; 109(1):19. https://doi.org/10.3390/ICC2024-17350
Chicago/Turabian StyleKull, Ann-Kathrin, and Dirk W. Lachenmeier. 2024. "Comprehensive Update on European Union Labeling Standards for Coffee and Its By-Products" Proceedings 109, no. 1: 19. https://doi.org/10.3390/ICC2024-17350
APA StyleKull, A.-K., & Lachenmeier, D. W. (2024). Comprehensive Update on European Union Labeling Standards for Coffee and Its By-Products. Proceedings, 109(1), 19. https://doi.org/10.3390/ICC2024-17350







