Optical Imaging of Intrinsic Neural Signals and Simultaneous MicroECoG Recording Using Polyimide Implants †
Abstract
:1. Introduction
2. Materials and Methods
2.1. ECoG Design, Fabrication and Packaging
2.2. Electrochemical and Optical Characterization
2.3. Animal Experiment
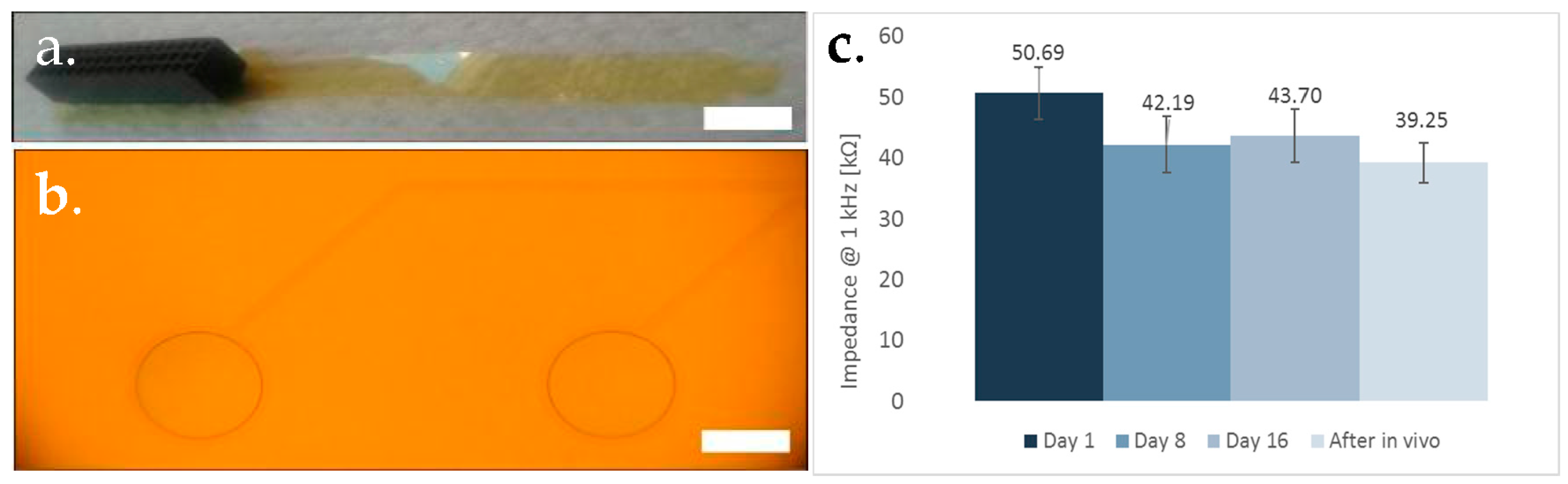
3. Results & Discussion
3.1. Optical Imaging
3.2. Electrical Recording
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Grinvald, A.; Lieke, E.; Frostig, R.D.; Gilbert, C.D.; Wiesel, T.N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 1986, 324, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Z.; Pongrácz, A. Multifunctional soft implants to monitor and control neural activity in the central and peripheral nervous system: A review. Sens. Actuators B Chem. 2017, 243, 1214–1223. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zátonyi, A.; Borhegyi, Z.; Cserpán, D.; Somogyvári, Z.; Srivastava, M.; Kisvárday, Z.; Fekete, Z. Optical Imaging of Intrinsic Neural Signals and Simultaneous MicroECoG Recording Using Polyimide Implants. Proceedings 2017, 1, 610. https://doi.org/10.3390/proceedings1040610
Zátonyi A, Borhegyi Z, Cserpán D, Somogyvári Z, Srivastava M, Kisvárday Z, Fekete Z. Optical Imaging of Intrinsic Neural Signals and Simultaneous MicroECoG Recording Using Polyimide Implants. Proceedings. 2017; 1(4):610. https://doi.org/10.3390/proceedings1040610
Chicago/Turabian StyleZátonyi, Anita, Zsolt Borhegyi, Dorottya Cserpán, Zoltán Somogyvári, Mohit Srivastava, Zoltán Kisvárday, and Zoltán Fekete. 2017. "Optical Imaging of Intrinsic Neural Signals and Simultaneous MicroECoG Recording Using Polyimide Implants" Proceedings 1, no. 4: 610. https://doi.org/10.3390/proceedings1040610
APA StyleZátonyi, A., Borhegyi, Z., Cserpán, D., Somogyvári, Z., Srivastava, M., Kisvárday, Z., & Fekete, Z. (2017). Optical Imaging of Intrinsic Neural Signals and Simultaneous MicroECoG Recording Using Polyimide Implants. Proceedings, 1(4), 610. https://doi.org/10.3390/proceedings1040610




