Development of a Novel Platelets Functional Assay Using QCM †
Abstract
:1. Introduction and Motivation
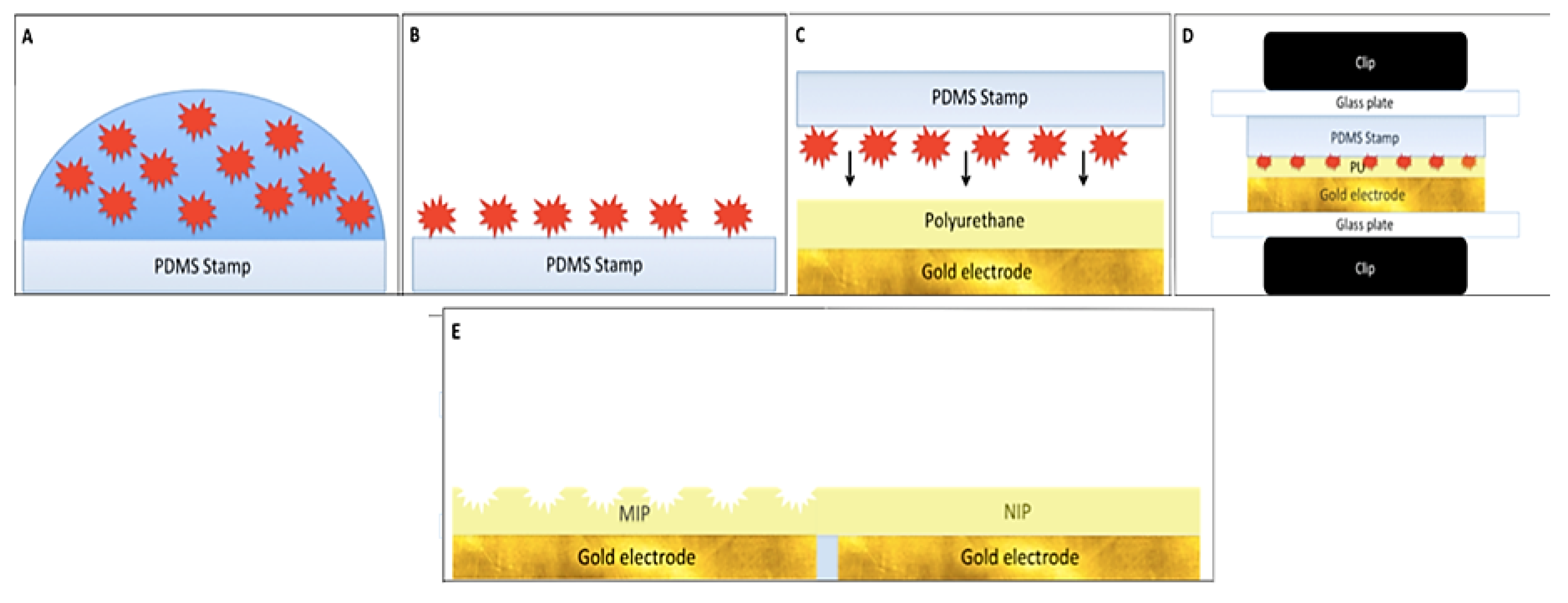
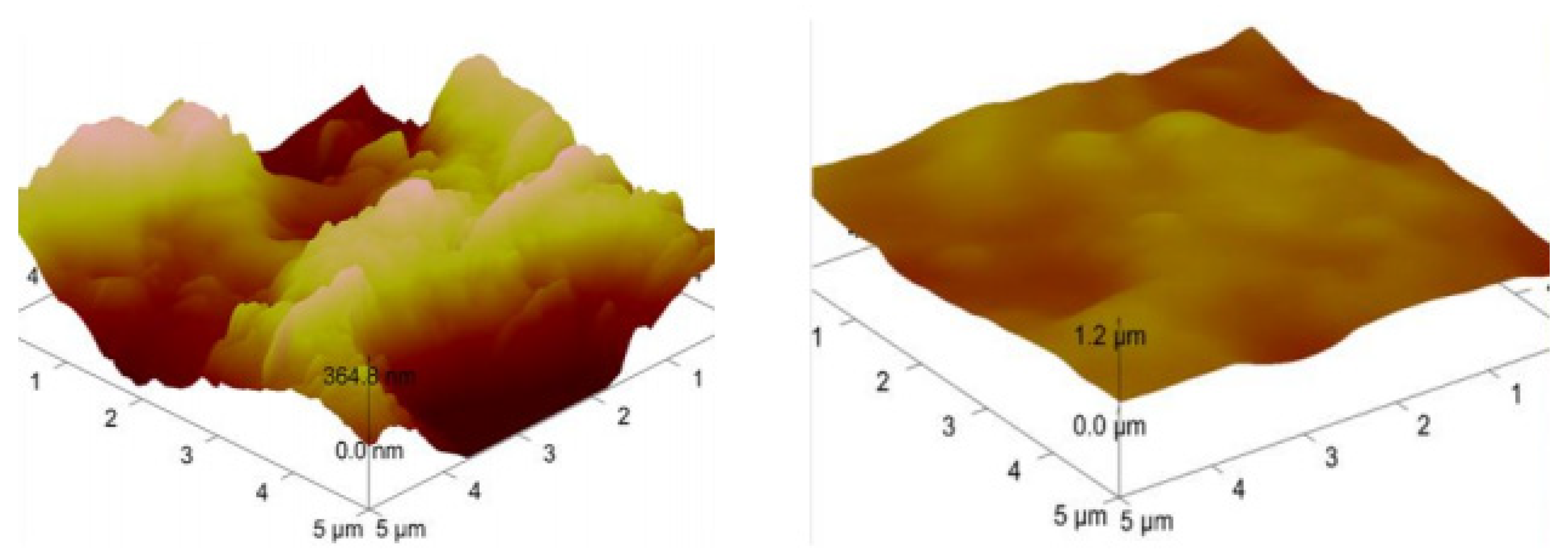
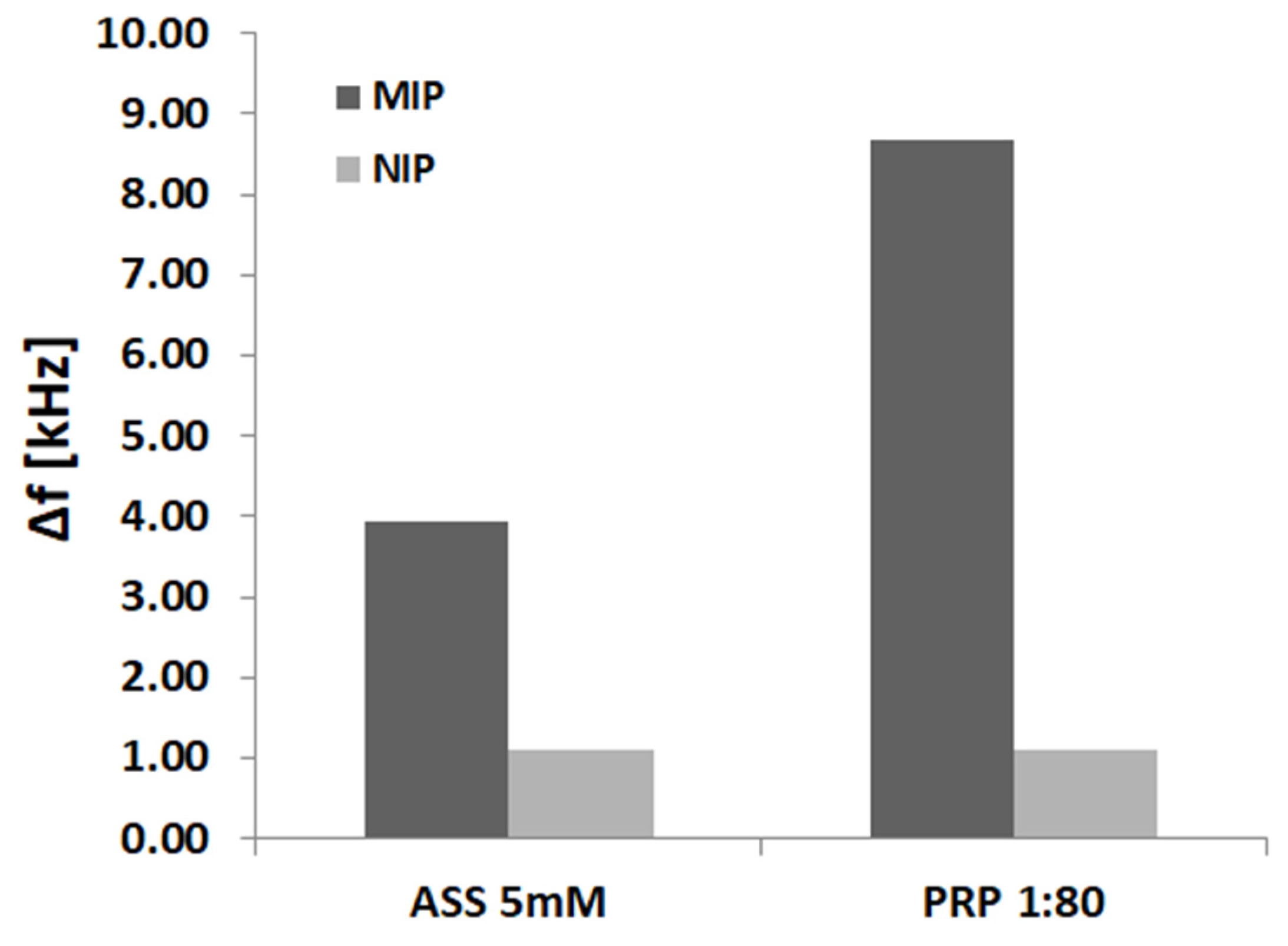
2. Methods and Results
3. Conclusions and Future Work
Conflicts of Interest
References
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.I. Effects of nonsteroidal anti-inflammatory drugs on platelet function and systemic hemostasis. J. Clin. Pharmacol. 1995, 35, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Israels, S.J. Laboratory testing for platelet function disorders. Int. J. Lab. Hematol. 2015, 37 (Suppl. 1), 18–24. [Google Scholar] [CrossRef]
- Kutner, C. Artificial Biosensors: How Can Molecular Imprinting Mimic Bio-recognition? Trends Biotechnol. 2016, 34, 922–941. [Google Scholar] [CrossRef]
- Seifner, A.; Lieberzeit, P.; Jungbauer, C.; Dickert, F.L. Synthetic receptors for selectively detecting erythrocyte ABO subgroups. Anal. Chim. Acta 2009, 651, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Hayden, O.; Mann, K.J.; Krassnig, S.; Dickert, F.L. Biomimetic ABO blood-group typing. Angew. Chem. Int. Ed. Engl. 2006, 45, 2626–2629. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strallhofer, A.E.; Jung, S.; Jungbauer, C.; Lieberzeit, P.A. Development of a Novel Platelets Functional Assay Using QCM. Proceedings 2017, 1, 514. https://doi.org/10.3390/proceedings1040514
Strallhofer AE, Jung S, Jungbauer C, Lieberzeit PA. Development of a Novel Platelets Functional Assay Using QCM. Proceedings. 2017; 1(4):514. https://doi.org/10.3390/proceedings1040514
Chicago/Turabian StyleStrallhofer, Agnieszka E., Stefan Jung, Christof Jungbauer, and Peter A. Lieberzeit. 2017. "Development of a Novel Platelets Functional Assay Using QCM" Proceedings 1, no. 4: 514. https://doi.org/10.3390/proceedings1040514
APA StyleStrallhofer, A. E., Jung, S., Jungbauer, C., & Lieberzeit, P. A. (2017). Development of a Novel Platelets Functional Assay Using QCM. Proceedings, 1(4), 514. https://doi.org/10.3390/proceedings1040514




