Integration and Bio-Functionalization of Vertically Aligned Carbon Nanotube Forests on High Frequency AlN Gravimetric Sensors †
Abstract
1. Introduction
2. Materials and Methods
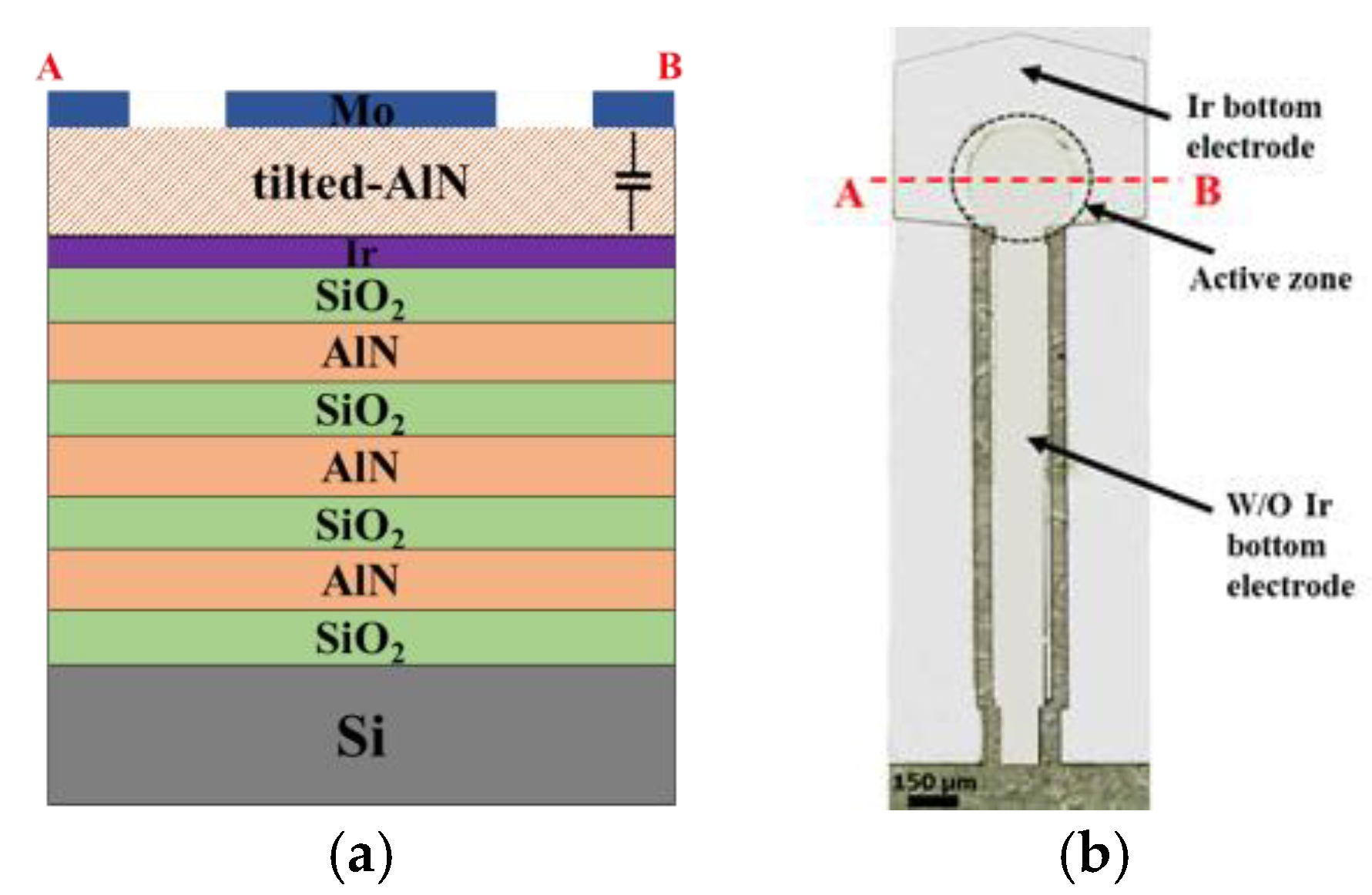
2.1. Device Fabrication
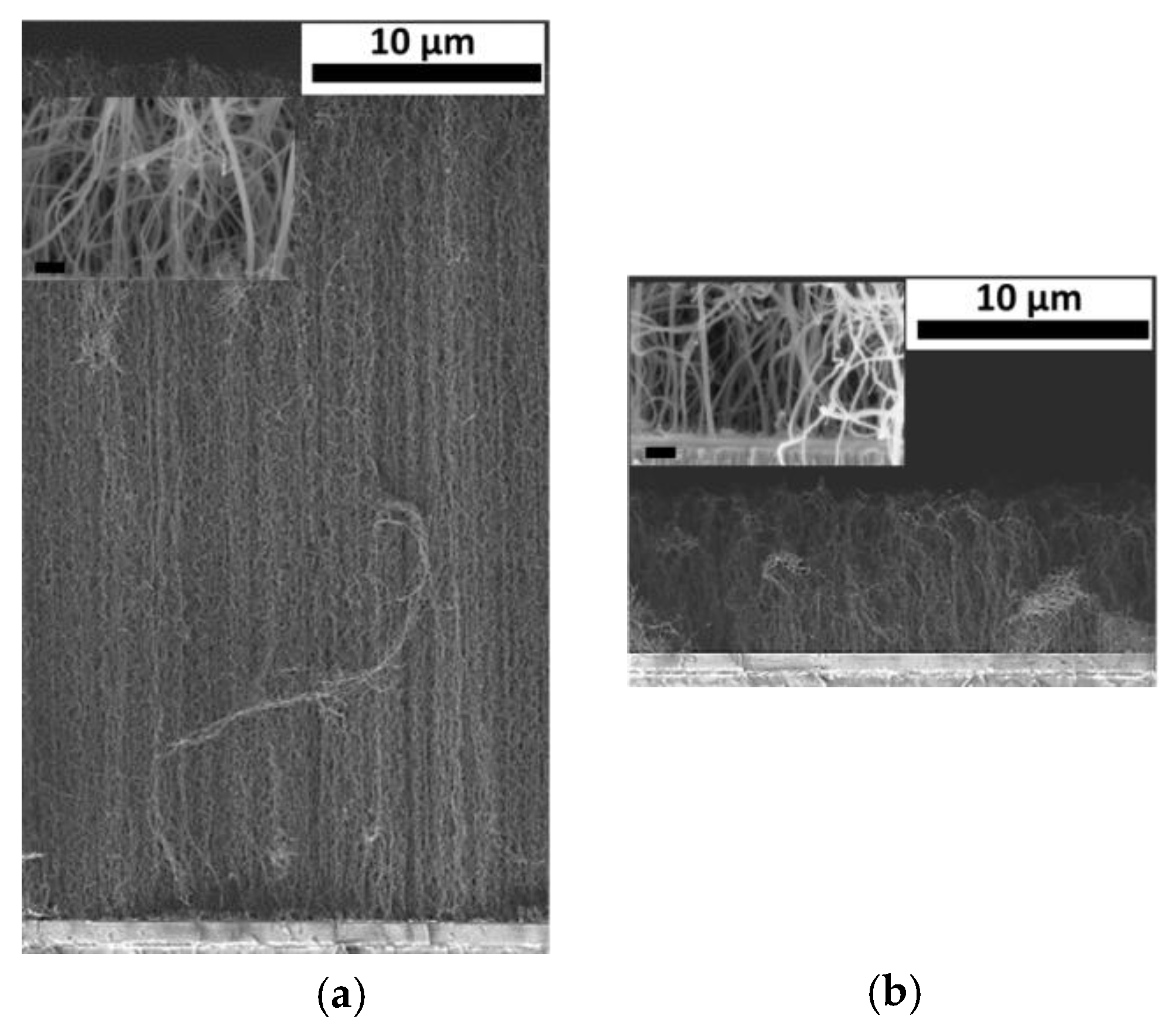
2.2. CNT Forests Integration
2.3. Functionalization of CNTs
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wagungdiinner Schichten und zur Mikrowagung. Zeitschrift fur Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Wingqvist, G.; Bjurstrom, J.; Hellgren, A.; Katardjiev, I. Immunosensor utilizing a shear mode thin film bulk acoustic sensor. Sens. Actuators B Chem. 2007, 127, 248–252. [Google Scholar] [CrossRef]
- DeMiguel-Ramos, M.; Díaz-Durán, B.; Escolano, J.-M.; Barba, M.; Mirea, T.; Olivares, J.; Clement, M.; Iborra, E. Gravimetric biosensor based on a 1.3GHz AlN shear-mode solidly mounted resonator. Sens. Actuators B Chem. 2017, 239, 1282–1288. [Google Scholar] [CrossRef]
- Van Hooijdonk, E.; Bittencourt, C.; Snyders, R.; Colomer, J.-F. Functionalization of vertically aligned carbon nanotubes. Beilstein J. Nanotechnol. 2013, 4, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Mirea, T.; Olivares, J.; DeMiguel-Ramos, M.; Clement, M.; Sangrador, J.; Iborra, E.; Esconjauregui, S. Carbon nanotube forests as top electrodes for AlN-based electroacoustic resonators. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 1476–1479. [Google Scholar]
- Olivares, J.; Mirea, T.; Díaz-Durán, B.; Clement, M.; DeMiguel-Ramos, M.; Sangrador, J.; de Frutos, J.; Iborra, E. Growth of carbon nanotube forests on metallic thin films. Carbon 2015, 90, 9–15. [Google Scholar] [CrossRef]
- Yost, A.L.; Shahsavari, S.; Bradwell, G.M.; Polak, R.; Fachin, F.; Cohen, R.E.; McKinley, G.H.; Toner, M.; Rubner, M.F.; Wardle, B.L. Layer-by-layer functionalized nanotube arrays: A versatile microfluidic platform for biodetection. Microsyst. Nanoeng. 2015, 1, 15037. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escolano, J.M.; Marco, B.; Mirea, T.; Olivares, J.; Clement, M.; Megias, D.; Iborra, E. Integration and Bio-Functionalization of Vertically Aligned Carbon Nanotube Forests on High Frequency AlN Gravimetric Sensors. Proceedings 2017, 1, 537. https://doi.org/10.3390/proceedings1040537
Escolano JM, Marco B, Mirea T, Olivares J, Clement M, Megias D, Iborra E. Integration and Bio-Functionalization of Vertically Aligned Carbon Nanotube Forests on High Frequency AlN Gravimetric Sensors. Proceedings. 2017; 1(4):537. https://doi.org/10.3390/proceedings1040537
Chicago/Turabian StyleEscolano, José Miguel, Bruno Marco, Teona Mirea, Jimena Olivares, Marta Clement, Diego Megias, and Enrique Iborra. 2017. "Integration and Bio-Functionalization of Vertically Aligned Carbon Nanotube Forests on High Frequency AlN Gravimetric Sensors" Proceedings 1, no. 4: 537. https://doi.org/10.3390/proceedings1040537
APA StyleEscolano, J. M., Marco, B., Mirea, T., Olivares, J., Clement, M., Megias, D., & Iborra, E. (2017). Integration and Bio-Functionalization of Vertically Aligned Carbon Nanotube Forests on High Frequency AlN Gravimetric Sensors. Proceedings, 1(4), 537. https://doi.org/10.3390/proceedings1040537



