In-Situ Electrophoretic Mobility Determination by Particle Image Velocimetry for Efficient Microfluidic Enrichment of Bacteria †
Abstract
:1. Introduction
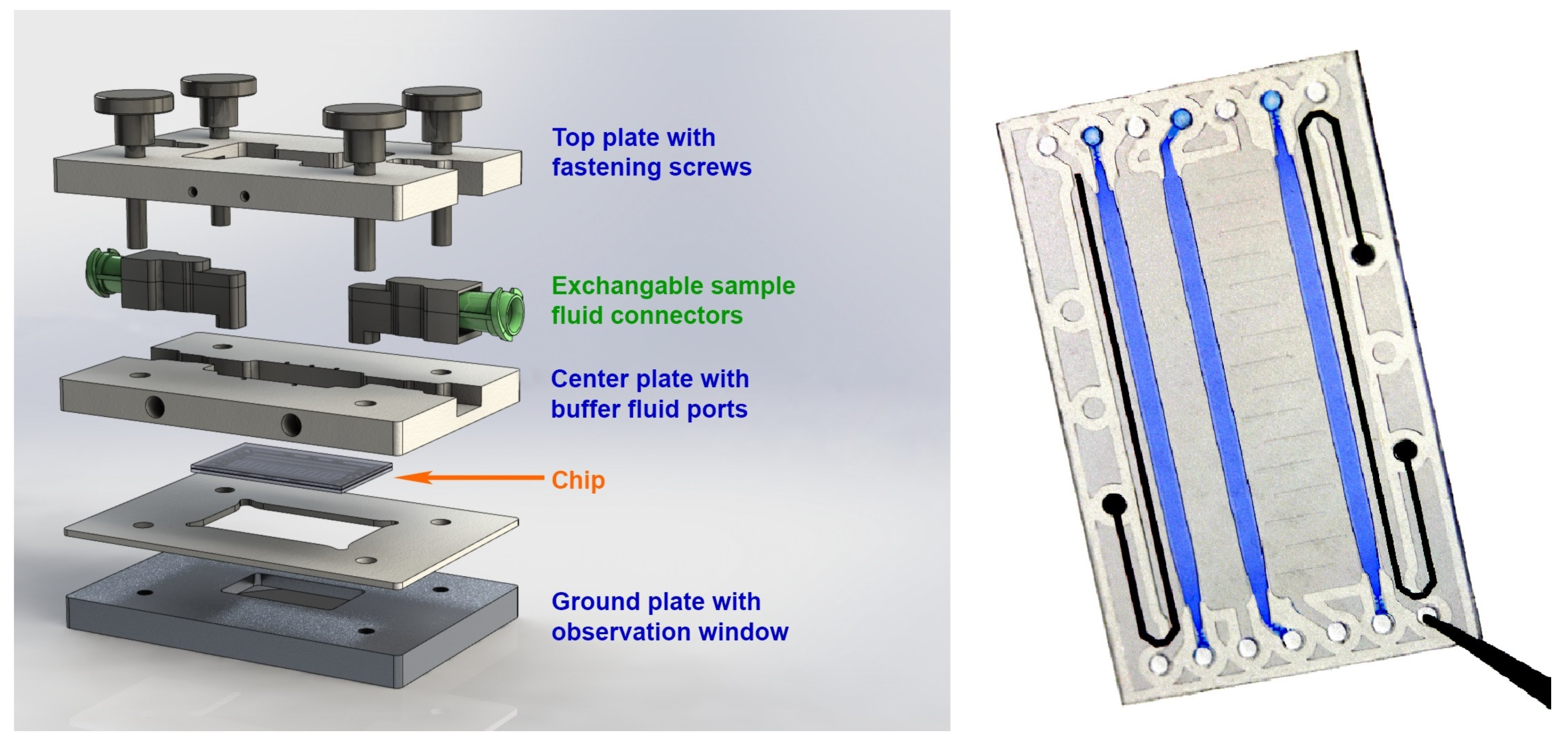
2. Materials and Methods
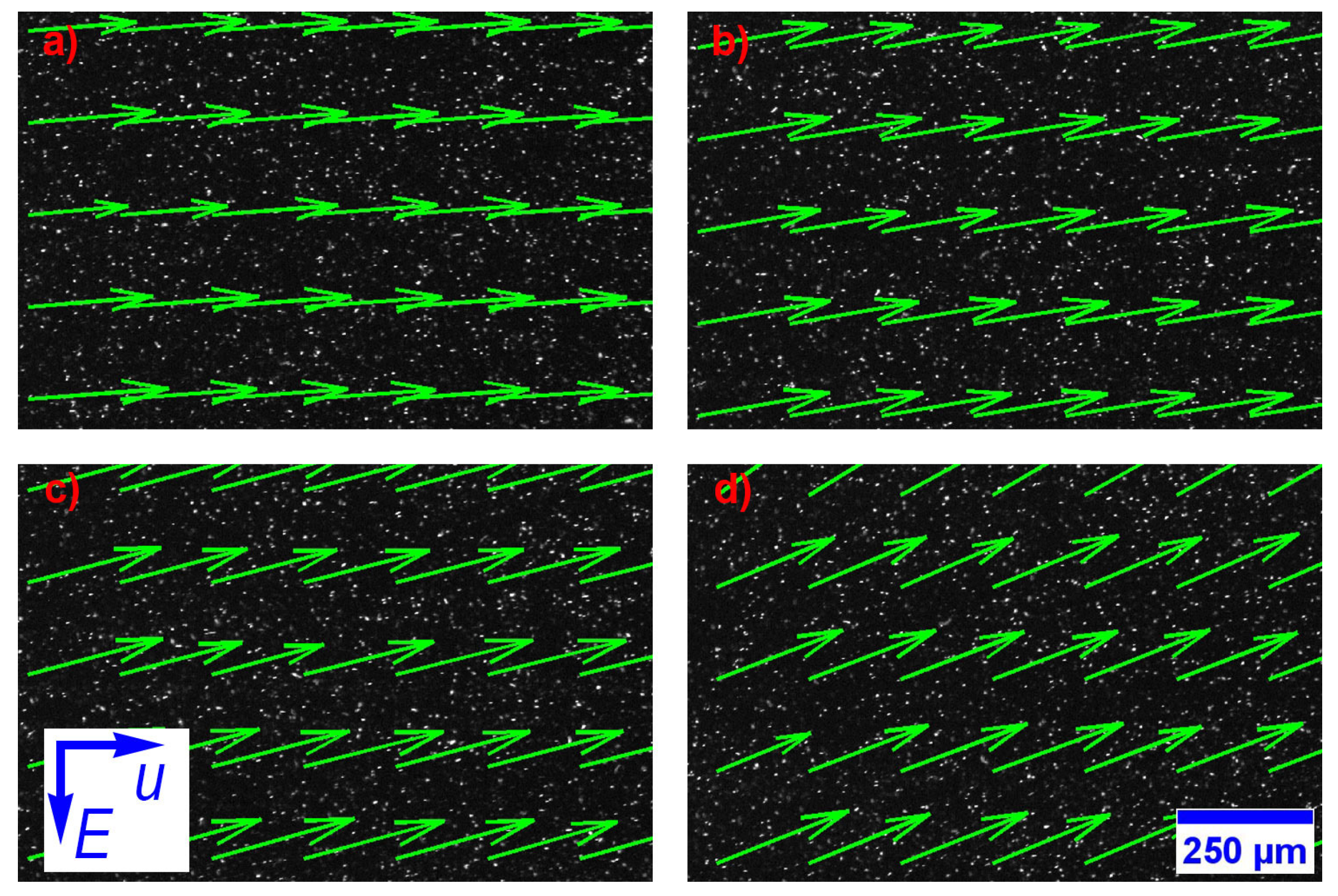
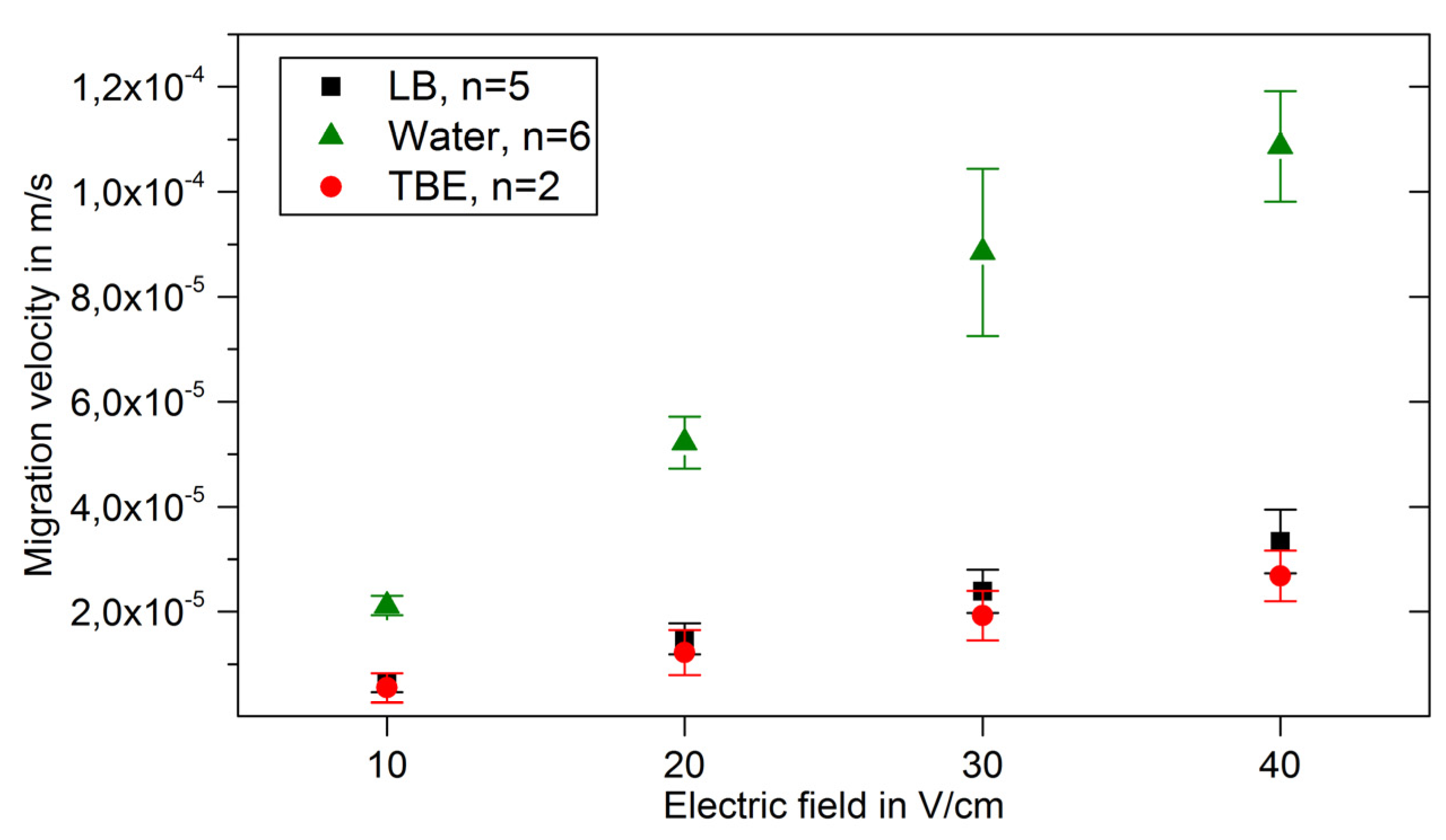
3. Results and Discussion
4. Conclusion and Outlook
Acknowledgments
Conflicts of Interest
References
- Podszun, S.; Vulto, P.; Heinz, H.; Hakenberg, S.; Hermann, C.; Hankemeier, T.; Urban, G.A. Enrichment of viable bacteria in a micro-volume by free-flow electrophoresis. Lab Chip 2012, 12, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Vulto, P.; Dame, G.; Maier, U.; Makohliso, S.; Podszun, S.; Zahn, P.; Urban, G.A. A microfluidic approach for high efficiency extraction of low molecular weight RNA. Lab Chip 2010, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Pedelacq, J.-D.; Cabantous, S.; Tran, T.; Terwilliger, T.C.; Waldo, G.S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 2006, 24, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Pfetsch, A.; Welsch, T. Determination of the electrophoretic mobility of bacteria and their separation by capillary zone electrophoresis. Fresenius J. Anal. Chem. 1997, 359, 198–201. [Google Scholar] [CrossRef]
- Adrian, R.J. Twenty years of particle image velocimetry. Exp. Fluids 2005, 39, 159–169. [Google Scholar] [CrossRef]
- Vulto, P.; Huesgen, T.; Albrecht, B.; Urban, G.A. A full-wafer fabrication process for glass microfluidic chips with integrated electroplated electrodes by direct bonding of dry film resist. J. Micromech. Microeng. 2009, 19, 77001. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Thielicke, W.; Stamhuis, E.J. PIVlab—Towards User-friendly, Affordable and Accurate Digital Particle Image Velocimetry in MATLAB. J. Open Res. Softw. 2014, 2, 1202. [Google Scholar] [CrossRef]
- Bayer, M.E.; Sloyer, J.L., Jr. The electrophoretic mobility of gram-negative and gram-positive bacteria: An electrokinetic analysis. J. Gen. Microbiol. 1990, 136, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Szumski, M.; Kłodzińska, E.; Dahm, H. Separation of bacteria by capillary electrophoresis. J. Sep. Sci. 2003, 26, 1045–1049. [Google Scholar] [CrossRef]
| Medium | Electrophoretic Mobility μ in m²/Vs | Electric Conductivity σ at 23 °C in μS/cm | pH |
|---|---|---|---|
| LB Luria | −0.75 × 10−8 ± 0.08 | 3500 | 7.0 |
| Tap water | −2.6 × 10−8 ± 0.35 | 510 | 8.5 |
| 1× TBE | −0.76 × 10−8 ± 0.16 | 1100 | 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rietzel, R.; Hügle, M.; Dame, G.; Behrmann, O.; Urban, G.A. In-Situ Electrophoretic Mobility Determination by Particle Image Velocimetry for Efficient Microfluidic Enrichment of Bacteria. Proceedings 2017, 1, 535. https://doi.org/10.3390/proceedings1040535
Rietzel R, Hügle M, Dame G, Behrmann O, Urban GA. In-Situ Electrophoretic Mobility Determination by Particle Image Velocimetry for Efficient Microfluidic Enrichment of Bacteria. Proceedings. 2017; 1(4):535. https://doi.org/10.3390/proceedings1040535
Chicago/Turabian StyleRietzel, Richard, Matthias Hügle, Gregory Dame, Ole Behrmann, and Gerald A. Urban. 2017. "In-Situ Electrophoretic Mobility Determination by Particle Image Velocimetry for Efficient Microfluidic Enrichment of Bacteria" Proceedings 1, no. 4: 535. https://doi.org/10.3390/proceedings1040535
APA StyleRietzel, R., Hügle, M., Dame, G., Behrmann, O., & Urban, G. A. (2017). In-Situ Electrophoretic Mobility Determination by Particle Image Velocimetry for Efficient Microfluidic Enrichment of Bacteria. Proceedings, 1(4), 535. https://doi.org/10.3390/proceedings1040535




