Characterization of Retinal Pigmented Epithelium Cells Density on a MicroElectrode Array Using Impedance Spectroscopy †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Growth
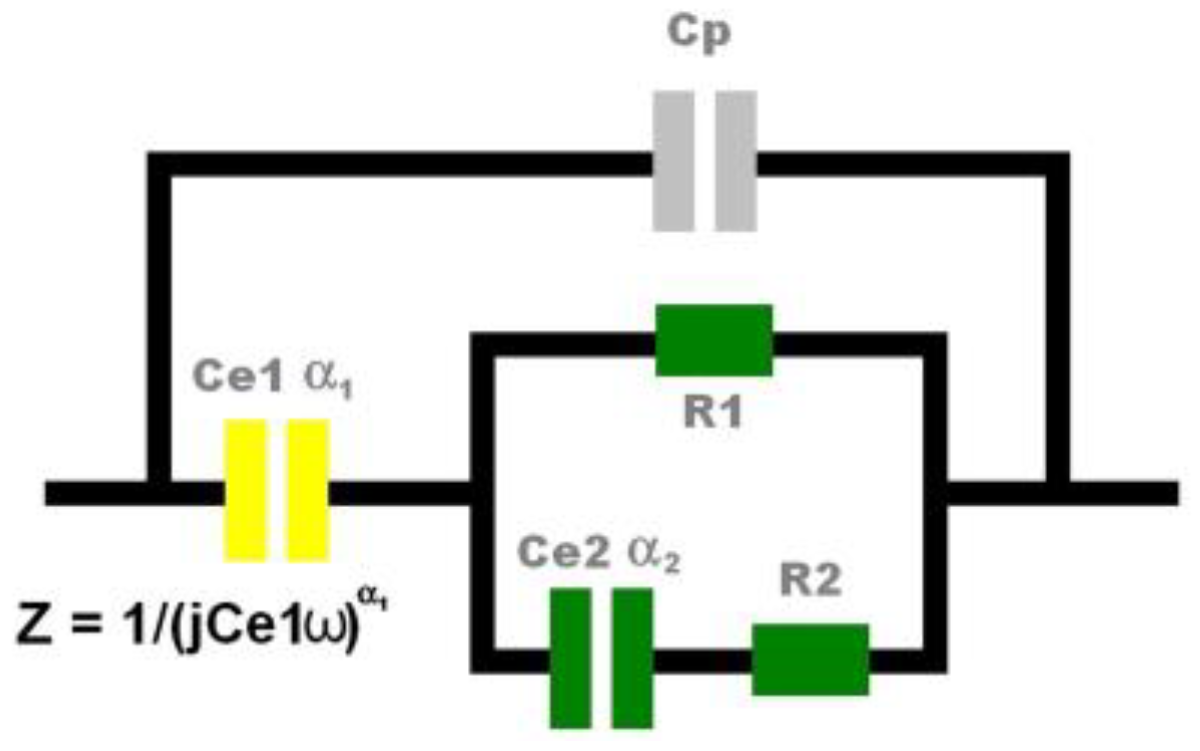
2.2. Electrical Model of the Cell Growth on MEA Device
3. Results
3.1. Cell Counting with Optical Measurement
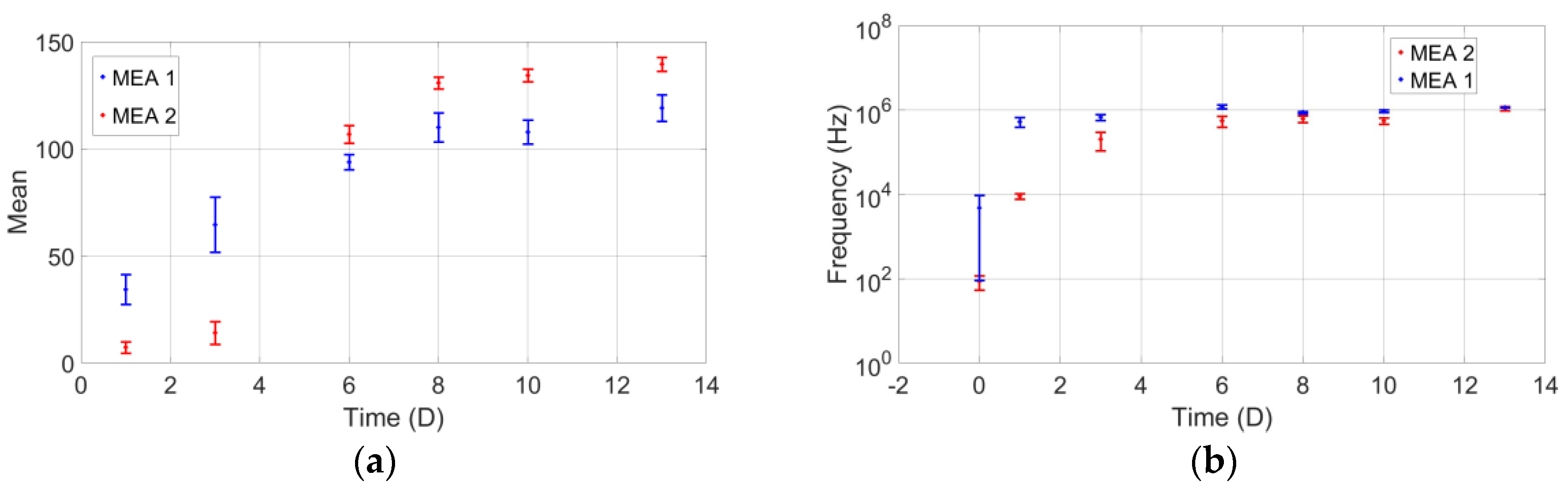
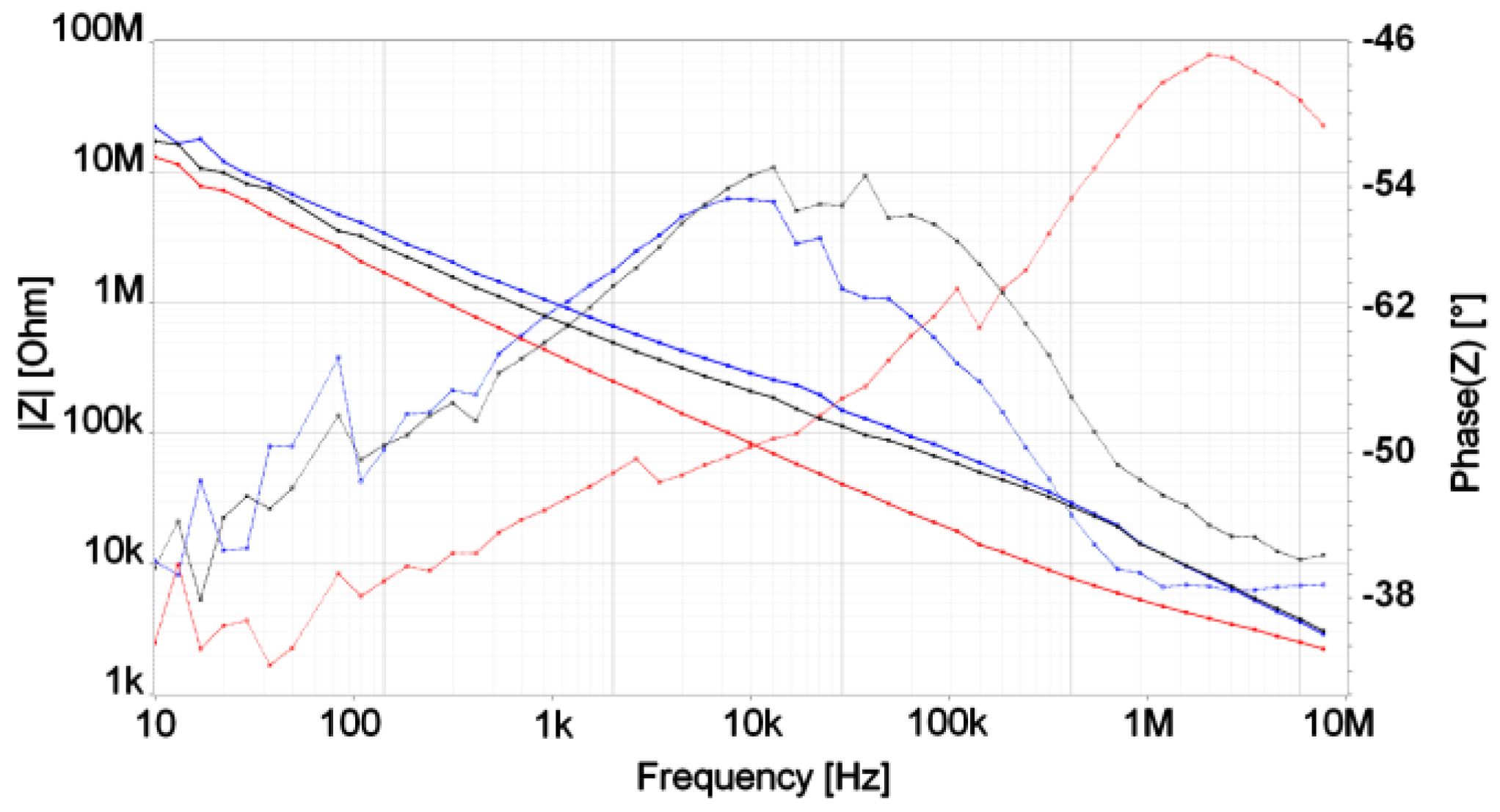
3.2. Electrical Measurements of Cell Growth
4. Discussion
Acknowledgments
Conflicts of Interest
References
- Frey, U.; Sedivy, J.; Heer, F.; Pedron, R.; Ballini, M.; Mueller, J.; Bakkum, D.; Hafizovic, S.; Faraci, F.D.; Greve, F.; et al. Switch-Matrix-Based High-Density Microelectrode Array in CMOS Technology. IEEE J. Solid State Circuits 2010, 45, 467–482. [Google Scholar] [CrossRef]
- Gamal, W.; Borooah, S.; Smith, S.; Underwood, I.; Srsen, V.; Chandran, S.; Bagnaninchi, P.O.; Dhillon, B. Real-time quantitative monitoring of hiPSC-based model of macular degeneration on Electric Cell-substrate Impedance Sensing microelectrodes. Biosens. Bioelectron. 2015, 71, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Picaud, S.; Sahel, J.-A. Retinal prostheses: Clinical results and future challenges. Comptes R. Biol. 2014, 337, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Pliquett, U. Bioimpedance: A Review for Food Processing. Food Eng. Rev. 2010, 2, 74–94. [Google Scholar] [CrossRef]
- Savolainen, V.; Juuti-Uusitalo, K.; Onnela, N.; Vaajasaari, H.; Narkilahti, S.; Suuronen, R.; Skottman, H.; Hyttinen, J. Impedance Spectroscopy in Monitoring the Maturation of Stem Cell-Derived Retinal Pigment Epithelium. Ann. Biomed. Eng. 2011, 39, 3055. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutzen, J.; Valet, M.; Fradot, V.; Rousseau, L.; Français, O.; Picaud, S.; Lissorgues, G. Characterization of Retinal Pigmented Epithelium Cells Density on a MicroElectrode Array Using Impedance Spectroscopy. Proceedings 2017, 1, 530. https://doi.org/10.3390/proceedings1040530
Boutzen J, Valet M, Fradot V, Rousseau L, Français O, Picaud S, Lissorgues G. Characterization of Retinal Pigmented Epithelium Cells Density on a MicroElectrode Array Using Impedance Spectroscopy. Proceedings. 2017; 1(4):530. https://doi.org/10.3390/proceedings1040530
Chicago/Turabian StyleBoutzen, Jocelyn, Manon Valet, Valérie Fradot, Lionel Rousseau, Olivier Français, Serge Picaud, and Gaëlle Lissorgues. 2017. "Characterization of Retinal Pigmented Epithelium Cells Density on a MicroElectrode Array Using Impedance Spectroscopy" Proceedings 1, no. 4: 530. https://doi.org/10.3390/proceedings1040530
APA StyleBoutzen, J., Valet, M., Fradot, V., Rousseau, L., Français, O., Picaud, S., & Lissorgues, G. (2017). Characterization of Retinal Pigmented Epithelium Cells Density on a MicroElectrode Array Using Impedance Spectroscopy. Proceedings, 1(4), 530. https://doi.org/10.3390/proceedings1040530




