Multiphysics Probe for Deep Brain Monitoring of Glioblastoma Environment †
Abstract
:1. Introduction
2. Materials and Methods
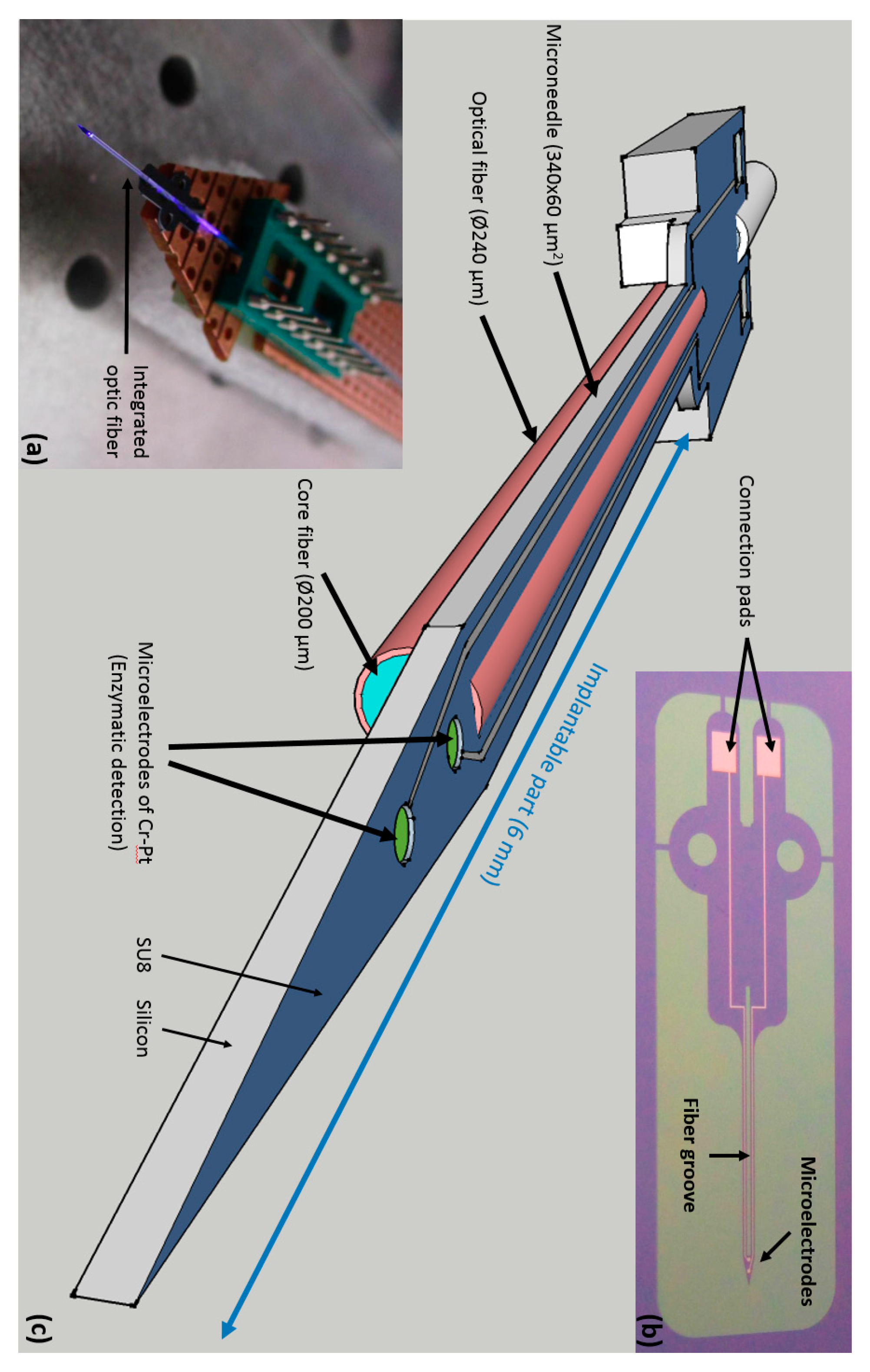
2.1. Probe Microfabrication
2.1.1. Design
2.1.2. Fabrication
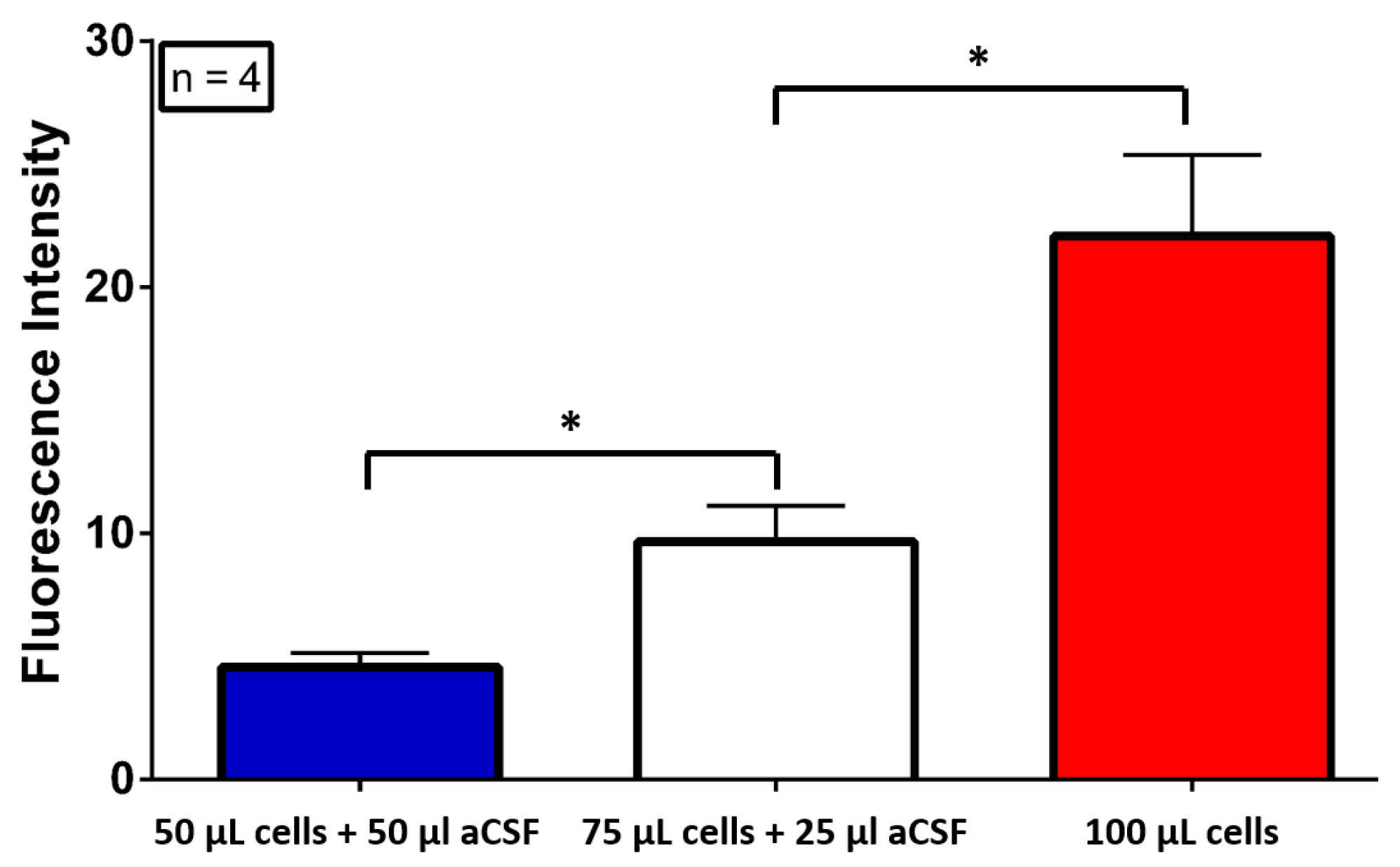
2.2. Fluorescence Monitoring
3. Results
4. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Omuro, A.; DeAngelis, L.M. Glioblastoma and other malignant gliomas: A clinical review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Maus, A.; Peters, G.J. Glutamate and α-ketoglutarate: Key players in glioma metabolism. Amino Acids 2017, 49, 21–32. [Google Scholar] [CrossRef]
- Szatmari, T.; Lumniczky, K.; Désaknai, S.; Trajcevski, S.; Hídvégi, E.J.; Hamada, H.; Sáfrány, G. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006, 97, 546–553. [Google Scholar] [CrossRef]
- Canales, A.; Jia, X.; Froriep, U.P.; Koppes, R.A.; Tringides, C.M.; Selvidge, J.; Lu, C.; Hou, C.; Wei, L.; Fink, Y.; et al. Multifunctional fibers for simultaneous optical, electrical, and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 2015, 33, 277–284. [Google Scholar] [CrossRef]
- Ozden, I.; Wang, J.; Lu, Y.; May, T.; Lee, J.; Goo, W.; O’Shea, D.J.; Kalanithi, P.; Diester, I.; Diagne, M.; et al. A coaxial optrode as multifunction write-read probe for optogenetic studies in non-human primates. J. Neurosci. Methods 2013, 219, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Vasylieva, N.; Marinesco, S.; Barbier, D.; Sabac, A. Silicon/SU8 multi-electrode micro-needle for in vivo neurochemical monitoring. Biosens. Bioelectron. 2015, 72, 148–155. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatard, C.; Pascual, O.; Jourlin, Y.; Marinesco, S.; Barbier, D.; Sabac, A. Multiphysics Probe for Deep Brain Monitoring of Glioblastoma Environment. Proceedings 2017, 1, 502. https://doi.org/10.3390/proceedings1040502
Chatard C, Pascual O, Jourlin Y, Marinesco S, Barbier D, Sabac A. Multiphysics Probe for Deep Brain Monitoring of Glioblastoma Environment. Proceedings. 2017; 1(4):502. https://doi.org/10.3390/proceedings1040502
Chicago/Turabian StyleChatard, Charles, Olivier Pascual, Yves Jourlin, Stéphane Marinesco, Daniel Barbier, and Andrei Sabac. 2017. "Multiphysics Probe for Deep Brain Monitoring of Glioblastoma Environment" Proceedings 1, no. 4: 502. https://doi.org/10.3390/proceedings1040502
APA StyleChatard, C., Pascual, O., Jourlin, Y., Marinesco, S., Barbier, D., & Sabac, A. (2017). Multiphysics Probe for Deep Brain Monitoring of Glioblastoma Environment. Proceedings, 1(4), 502. https://doi.org/10.3390/proceedings1040502





