Abstract
An electrochemical microsensor system to monitor the pericellular oxygen concentration of fibroblasts during low-level light therapy in vitro was developed. The system provides in-sight into the metabolism of the cells during and in consequence of illumination with visible red light. This approach is a unique method for real-time investigations of cellular respiration during light therapy. The presented sensor system features direct amperometric measurements by using chronoamperometric protocols for long-term stability. The oxygen measurements do not show a disturbance by light.
1. Introduction
Low level light therapy (LLLT), also termed photobiomodulation, mainly in the red and near-infrared range has been shown to induce stimulatory effects in wound healing and tissue regeneration. Irradiation with light emitting diodes (LEDs) leads to an increased cell proliferation as well as to a reduced time of closure of a wound [1]. Although the success in treatment of wounds by visible red light has been already shown in several in vivo studies [2], the corresponding intracellular mechanisms during and after the therapy are only partially understood. It is speculated, that the stimulation of mitochondrial activity by light, however, could be one of the main underlying processes. So far, there is no in vitro platform to monitor metabolic effects of low-level light therapy continuously. Until now, evaluation is performed by single-point measurements only. Due to the presence of the oxygen concentration gradient along the medium height, it is important to place sensors in the direct vicinity of the cells (especially in 2D cell cultures) to obtain true pericellular readings and to deduce respiration rates [3]. The presented platform allows the integration of other sensors, such as glucose and lactate biosensors, to enable metabolic monitoring during light therapy.
2. Experimental
The presented system combines pericellular measurements during light therapy as well as on-chip cell culturing. Hence, our approach facilitates investigations of different therapeutic strategies by metabolic microsensors in vitro. We developed a platform with electrochemical sensors and illumination by visible red light. Transparent sensor chips were integrated in a cell culture well with a diameter of 10 mm to allow cell culturing directly on the sensor chip surface (Figure 1a). The sensor features a three-electrode setup containing a platinum working and counter electrode and a silver/silverchloride electrode as reference. All electrodes are covered with a pHEMA-based hydrogel as diffusion limiting membrane [3]. Long-term stability, even in cell culture medium containing 10% fetal calf serum, was achieved by a chronoamperometric protocol comprising three steps.
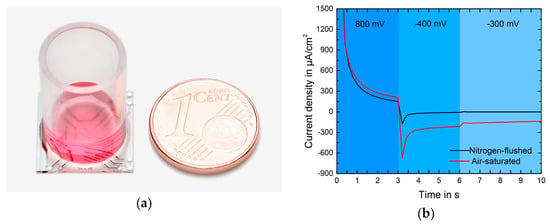
Figure 1.
(a)Transparent electrochemical sensor chip with cell culture well (transparent plastic cylinder); (b) Current response of one cycle. The 3-step protocol enables cleaning of the electrode surface.
- Step I: Formation of PtO (0.8 V vs. on-chip RE).
- Step II: Reduction of PtO (−0.4 V).
- Step III: Measurement of oxygen (−0.3 V).
In Figure 1b the current responses in medium are shown for air-saturated and nitrogen-flushed solution. Oxygen concentration was measured every 6 minutes. The last three seconds of a cycle were averaged and present one data point [3]. An embryonic mouse fibroblast (NIH-3T3) cell line was used for this study. Cells were treated with pulsing red LED light (635 nm) using REPULS (Repuls Lichtmedizintechnik GmbH, Vienna, Austria) at a mean power intensity of 50 mW/cm2. The LED device was mounted 10 cm above the measurement platform.
3. Results
Oxygen was measured in cell culture before, during and after light exposure. The sensors were calibrated at 37 °C in cell culture medium equilibrated from 0.5 to 20.95% in the gas phase which corresponds to 1.6 to 198.2 μM dissolved oxygen. The sensitivity of the oxygen sensors was found to be −0.63 µAcm−2µM−1 and the offset was less than −1 µAcm−2 (Figure 2a). A linear dependency between current density and oxygen concentration was observed. This allowed for a 1-point calibration scheme during cell culture measurements. It was shown, that the sensors were longtime stable in cell culture medium comprising serum (Figure 2b).
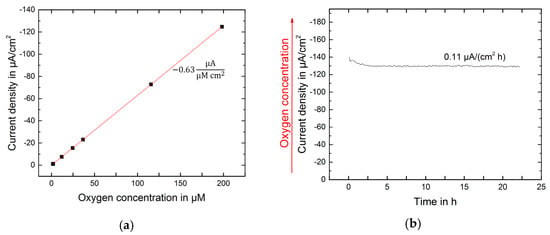
Figure 2.
(a) Calibration of the oxygen sensors in cell culture medium at 37 °C; (b) Oxygen measured in cell culture medium in the incubator at 37 °C.
Embryonic mouse fibroblasts were successfully cultured on the sensor chip surface (Figure 3). Optical inspection did not reveal any morphological deviation from cells in standard cell culture labware.
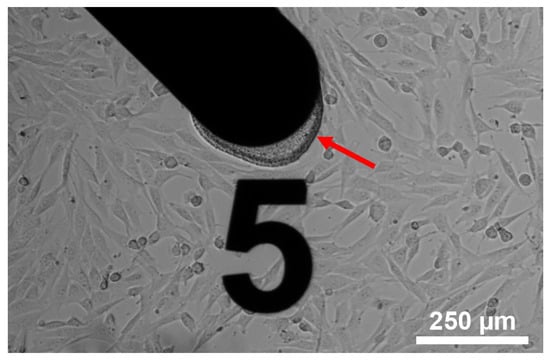
Figure 3.
NIH-3T3 cells cultivated on the sensor chip surface. The arrow indicates the pHEMA based hydrogel on the working electrode.
The respiration of the fibroblasts was monitored over three days. Low-level light therapy using REPULS was applied on the first and second day. First measurements indicate an overall increase in respiration immediately after both stimulations compared to not illuminated control. After the second illumination the decrease in the pericellular oxygen was even higher over time (Figure 4a). A second measurement of pericellular oxygen without cells was performed during low-level light therapy. After 21 h four illuminations were applied. Even though small peaks in the measurements appear due to the handling of the setup a change in the oxygen concentration can not be observed (Figure 4b).
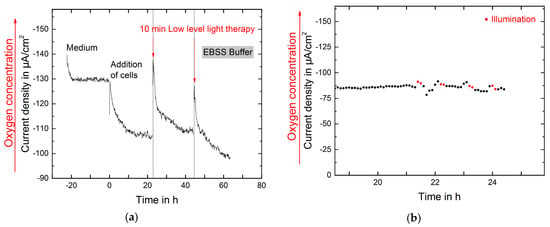
Figure 4.
(a) Monitoring of the respiration of NIH-3T3 cells over three days. On two consecutive days low-level light therapy was applied and in consequence the oxygen concentration decreased significantly; (b) Oxygen measured in EBSS Buffer at room temperature: after 21 h four illuminations à 10 min take place.
4. Conclusions and Outlook
The presented microsensor system allows the measurement of metabolic parameter during and after light therapy in cell culture. In order to provide on-chip treatment NIH-3T3 cells were successfully cultured in the cell culture chamber integrated on the platform. Pericellular oxygen concentrations were monitored by the use of electrochemical sensors. The system shows a longtime stable signal. The measurements indicate that an overall increase in cell respiration can be observed during and after light therapy. Further investigations, using different types of biosensors should clarify the influence of light therapy on cell metabolism.
Acknowledgments
We would like to thank the “Freunde der Universität Freiburg e.V.” for the support with travel costs and Repuls Lichtmedizintechnik GmbH, Vienna, Austria, for the support by providing the light source.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Teuschl, A.; Balmayor, E.R.; Redl, H.; van Griensven, M.; Dungel, P. Phototherapy with LED light modulates healing processes in an in vitro scratch model using 3 different cell types. Dermatol. Surg. 2015, 41, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Dungel, P.; Hartinger, J.; Chaudary, S.; Slezak, P.; Hofmann, A.; Hausner, T.; Strassl, M.; Wintner, E.; Redl, H.; Mittermayr, R. Low level light therapy by LED of different wavelength induces angiogenesis and improves ischemic wound healing. Lasers Surg. Med. 2014, 46, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kieninger, J.; Aravindalochanan, K.; Sandvik, J.A.; Pettersen, E.O.; Urban, G.A. Pericellular oxygen monitoring with integrated sensor chips for reproducible cell culture experiments. Cell Prolif. 2014, 47, 180–188. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).