Multimodal Neuroimaging Microtool for Infrared Optical Stimulation, Thermal Measurements and Recording of Neuronal Activity in the Deep Tissue
†
Abstract
:1. Introduction
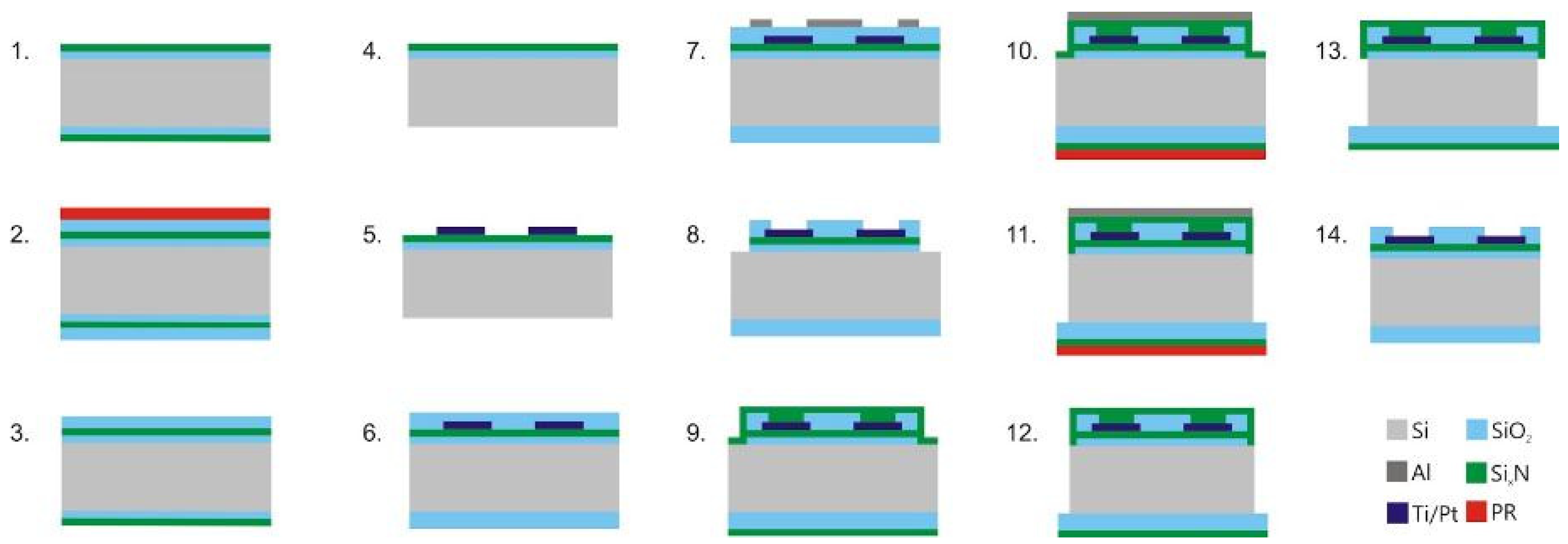
2. Design and Technology
3. Test Methods
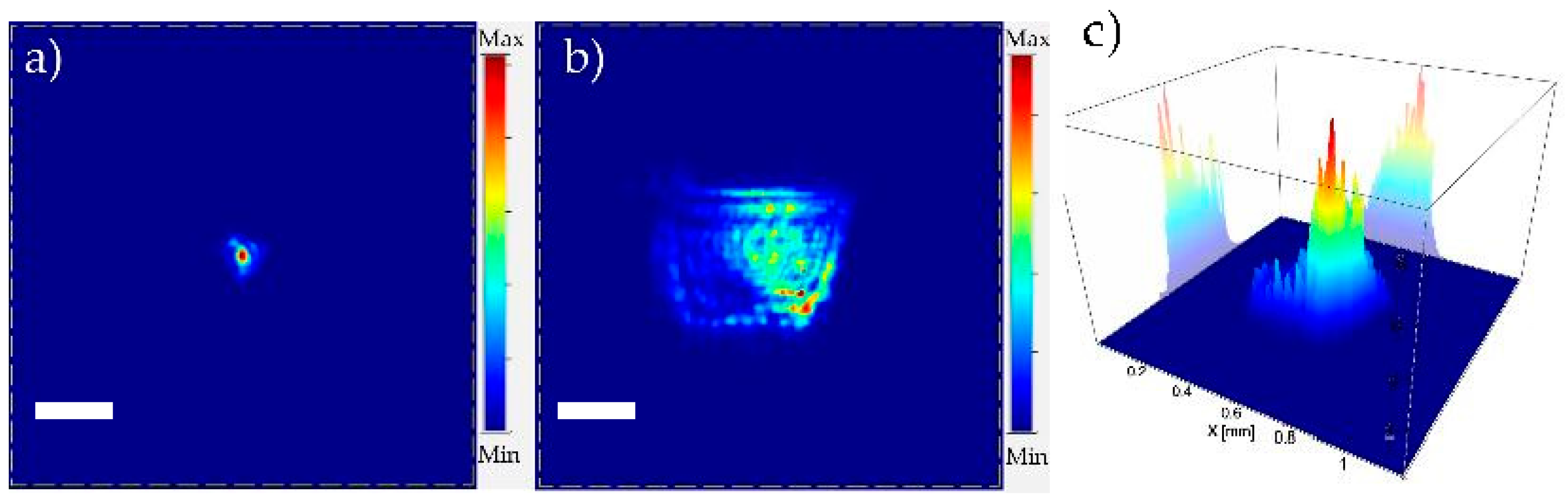
3.1. Optical Characterization
3.2. Thermal Characterization
3.3. Electrical Characterization
Acknowledgments
Conflicts of Interest
References
- Wells, J.; Kao, C.; Jansen, E.D.; Konrad, P.; Mahadevan-Jansen, A. Application of infrared light for in vivo neural stimulation. J. Biomed. Opt. 2005, 10, 064003. [Google Scholar] [CrossRef] [PubMed]
- Kiss, M.; Földesy, P.; Fekete, Z. Optimization of a Michigan-type silicon microprobe for infrared neural stimulation. Sens. Actuators B Chem. 2016, 224, 676–682. [Google Scholar] [CrossRef]
- Fekete, Z.; Csernai, M.; Kocsis, K.; Horváth, Á.C.; Pongrácz, A.; Barthó, P. Simultaneous in vivo recording of local brain temperature and electrophysiological signals with a novel neural probe. J. Neural Eng. 2017, 14, 034001. [Google Scholar] [CrossRef] [PubMed]


| Parameter (dim.) | Value |
|---|---|
| Shaft width (μm) | 170 |
| Shaft thickness (μm) | 190 |
| Shaft length (mm) | 5 |
| Recording site area (μm2) | 900 |
| Number of recording sites | 4–16 |
| Zsite at 1 kHz (kΩ) | 1031 ± 95 |
| αTsensor (ppm/K) | 2636 ± 75 |
| Detection limit (°C) | 0.14 |
| Thermal time constant (ms) | 472 |
| Waveguide efficiency (%) | 32.04 ± 4.10 |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horváth, Á.C.; Sepsi, Ö.; Boros, C.Ö.; Beleznai, S.; Koppa, P.; Fekete, Z.
Multimodal Neuroimaging Microtool for Infrared Optical Stimulation, Thermal Measurements and Recording of Neuronal Activity in the Deep Tissue
. Proceedings 2017, 1, 494.
https://doi.org/10.3390/proceedings1040494
Horváth ÁC, Sepsi Ö, Boros CÖ, Beleznai S, Koppa P, Fekete Z.
Multimodal Neuroimaging Microtool for Infrared Optical Stimulation, Thermal Measurements and Recording of Neuronal Activity in the Deep Tissue
. Proceedings. 2017; 1(4):494.
https://doi.org/10.3390/proceedings1040494
Horváth, Ágoston C., Örs Sepsi, Csanád Ö. Boros, Szabolcs Beleznai, Pál Koppa, and Zoltán Fekete.
2017. "Multimodal Neuroimaging Microtool for Infrared Optical Stimulation, Thermal Measurements and Recording of Neuronal Activity in the Deep Tissue
" Proceedings 1, no. 4: 494.
https://doi.org/10.3390/proceedings1040494
Horváth, Á. C., Sepsi, Ö., Boros, C. Ö., Beleznai, S., Koppa, P., & Fekete, Z.
(2017). Multimodal Neuroimaging Microtool for Infrared Optical Stimulation, Thermal Measurements and Recording of Neuronal Activity in the Deep Tissue
. Proceedings, 1(4), 494.
https://doi.org/10.3390/proceedings1040494





