1. Introduction
The demand of real time, low cost and small size sensors is continuously increasing to control a large variety of parameters such as environmental, food quality, biomedical, etc. In the context of gas sensors, great advantages have been achieved through an extensive investigation on the semiconductor metal oxides (MOX) benefiting from progresses in nano-technology, recently occurred. In particular, the detection at very low concentration of gases is of paramount importance for medical and environmental applications to detect gases such as acetone, ethanol, isoprene and benzene.
The aim of this work is to develop nanomaterials for high sensitivity gas sensors. TiO
2 and ZnO have been selected because they have been widely investigated in solar cells, photocatalysts and gas sensors [
1]. TiO
2 and ZnO can be obtained in form of nanostructures via physical or chemical processes in different morphologies. Among these techniques, electrospinning and sol-gel methods have been adopted to compare their morphological, structural, optical and functional properties.
Electrospinning represents a new, simple and versatile method for generating ceramic porous nanofibers with interconnective pores and high specific surface area [
2]. The sol-gel synthesis is a traditional method to prepare nanopowders with tailored properties, in terms of purity, morphology and grain size homogeneity.
The thick films produced from the synthesized nanopowders have been measured trough dynamical responses in presence of the target gases. Moreover, Arrhenius plots and energy barriers versus temperature in dry air as well as FTIR analysis (performed onto ZnO materials during interaction with acetone at the same working temperatures of the sensors) have been carried out in order to investigate on the Schottky barrier model and on the electronic properties of the investigated materials.
2. Materials and Methods
Solutions for electrospinning were prepared by mixing: (i) ethanol (55.6 wt %), PVP (9.5 wt %), acetic acid (23.6 wt %) and Ti (IV) butoxide (11.2 wt %); (ii) ethanol (28 wt %), PVP (16 wt %), water (25 wt %) and zinc nitrate (32 wt %). For both Ti and Zn precursor solutions, the electrospinning process was performed in air, at RT with a feeding rate of 10 μL/min, a voltage of 30 kV and a working distance of 14 cm. The generated fibers underwent calcination at 450 °C obtaining TiO2 and ZnO (here named ES). Starting from the same precursors, ZnO and TiO2 were synthesized through a sol-gel route (here named SG). Ti (IV) n-butoxide was dissolved in absolute ethanol (0.23 M) and added to a solution of ethanol/water 1:1 vol. For ZnO, a proper amount of ammonium hydroxide was added to a water solution 0.05 M of Zn(NO3)2·6H2O to reach a pH of 10 and ageing the solution at RT for 1 h. The precipitates, (Ti(OH)4) and ε-Zn(OH)2, were filtered, washed, dried and finally calcined at 400 and 450 °C in air for 2 h, respectively obtaining anatase TiO2 and ZnO.
The sensing films were deposited through screen-printing technique onto miniaturized alumina substrates each one provided with a heater element and interdigitated contacts, firing all samples at 650 °C. Hereinafter, the TiO2 films will be named TES, if obtained by electrospinning, and TSG by sol-gel; the same for ZnO films, ZES and ZSG, respectively.
X-ray diffraction (XRD) patterns of the samples were collected to determine the crystalline phases and the average crystallite sizes by applying the Scherrer’s formula on the (101) diffraction peak. The specific surface areas were determined through the Brunauer-Emmett-Teller (BET) method to the adsorption/desorption isotherms of N2 at 77 K. Morphological characterization of the samples was performed by Field Emission Scanning Electron Microscopy (FE-SEM).
Absorption IR spectra were run on the powder samples compressed in self-supporting discs and placed in a commercial heated stainless steel cell allowing thermal treatments in situ under vacuum or controlled atmosphere. The IR spectra were recorded for ZnO during the interaction with a mixture acetone/O2 (1:5, pacetone = 5 mbar) at increasing temperature up to 400 °C.
The flow-through technique was used to test the electrical properties of the sensors, at a flow rate of 0.5 L/min using synthetic air as carrier gas. Conductance as a function of temperature (300–900 K) and surface barrier height (carried out using the method of temperature-stimulated conductance measurements) were measured to determine the intergranular energy barrier versus temperature. Moreover, dynamic responses of sensing films were obtained in presence of mixture of different gases varying the operating temperature from 350 to 550 °C, defining the sensor response as ratio between the conductance in presence of the target gas and the conductance in air.
3. Results and Discussion
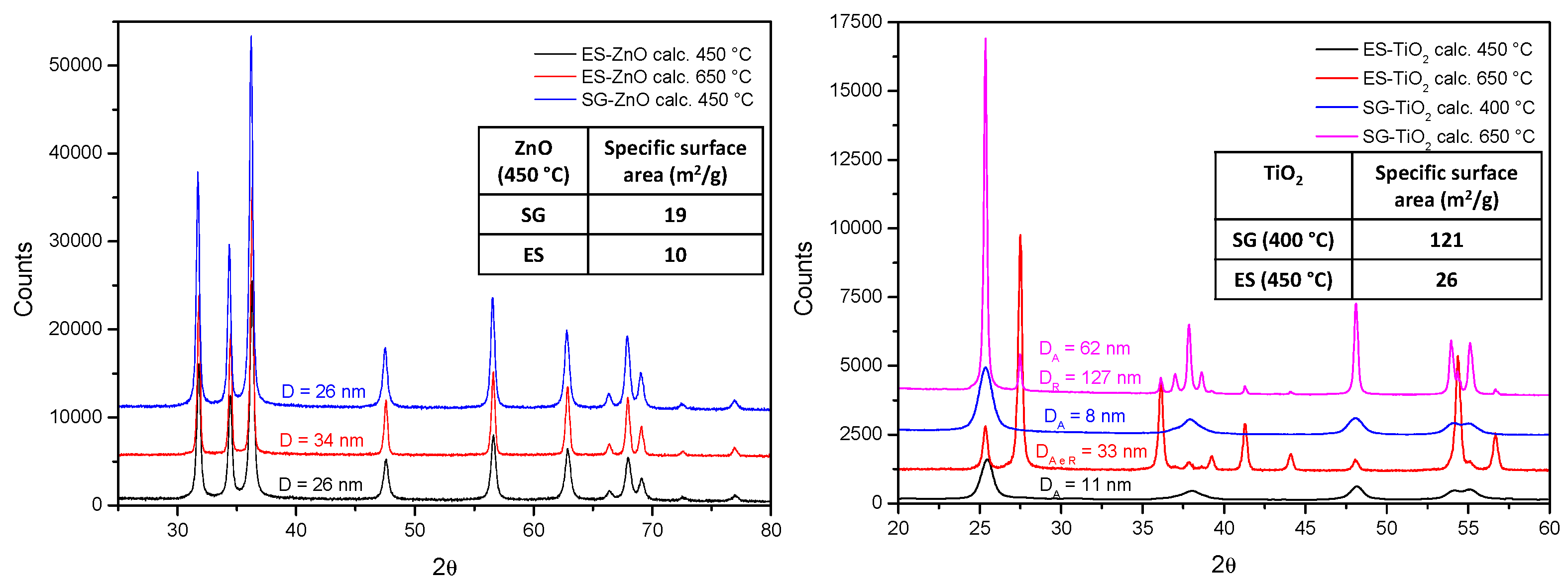
XRD analysis showed a hexagonal wurtzite structure for ES and SG-ZnO and anatase phase for ES and SG-TiO
2 calcined at 450 °C. Both anatase and rutile phase are present calcining at 650 °C (
Figure 1).
For both ZnO and TiO2, average crystallite size of the material obtained via electrospinning is comparable to that of the material obtained via sol-gel. This is not reflected on the specific surface areas (see Table 1). In particular, SG materials show surface areas higher than the corresponding ES materials. This is related to the presence of meso-pores in the SG samples, which volumes are 0.0986 cm3/g and 0.2922 cm3/g for SG-ZnO and SG-TiO2, respectively. The mesoporosity is particularly marked for SG-TiO2 and this justifies the surface area markedly higher with respect to ES-TiO2.
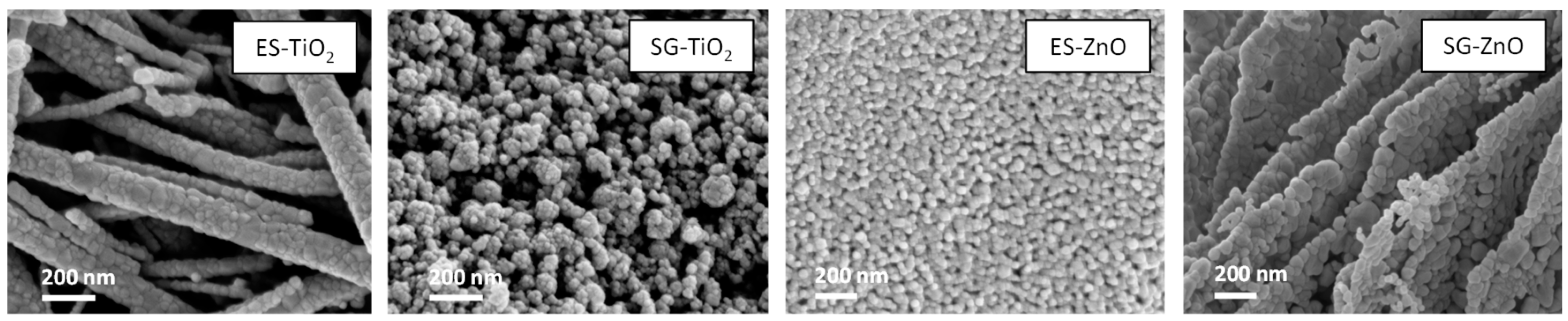
In
Figure 2, the SEM images reveal that the ES-TiO
2 sample retains, after the thermal treatment, the fibrous nature characteristic of the as-electrospun material. The fibers are not single crystals but are particle aggregates. SG-TiO
2 are constituted by globular particle aggregates observable also for the ZnO samples; the particles of ES-ZnO are smaller than that of SG-ZnO. It is worth of note that ES-ZnO does not retain the fibrous morphology shown by ES-TiO
2.
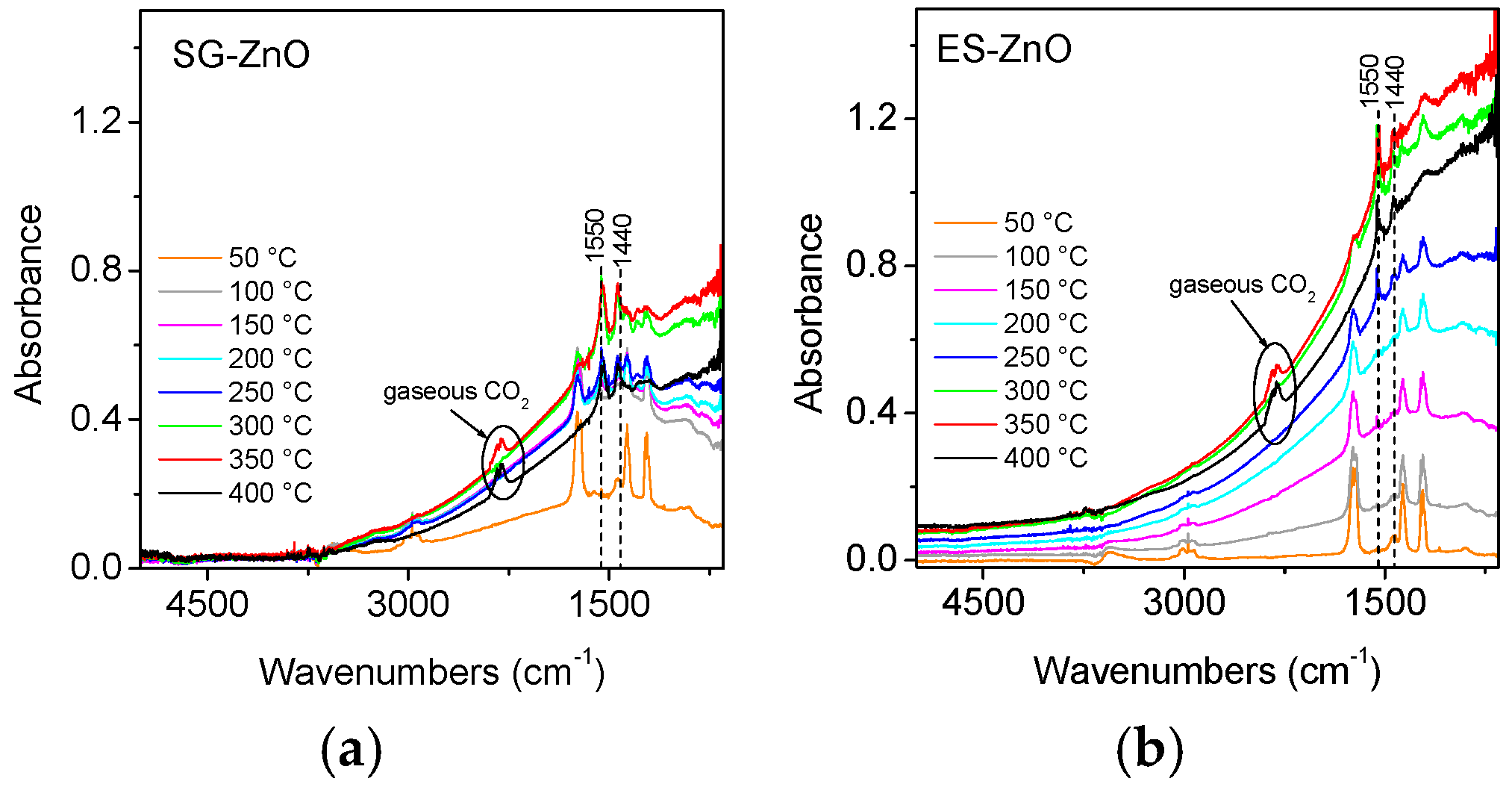
IR absorption spectra of ZnO materials in interaction with a mixture acetone/O
2 at increasing temperature are reported in
Figure 3. The spectra of both ZnO samples are characterized by the increase of the electronic absorption related to the transition of electrons from mono-ionized oxygen vacancies to the conduction band up to 350 °C. At 400 °C the electronic absorption band decreases due to partial re-oxidation of the surface caused by oxygen in the mixture. The bands of the gaseous acetone are superimposed to the broad electronic band and decrease in intensity on increasing temperature, being acetone consumed by the surface reactions. Surface carbonates (bands at 1440 and 1550 cm
−1) are formed by the chemisorption of CO
2 due to the acetone oxidation. The integrated intensity of the electronic absorption exhibits higher value for the ES-ZnO than for SG-ZnO, in agreement with electrical measurements that reveal higher response to acetone for ES than for SG sample.
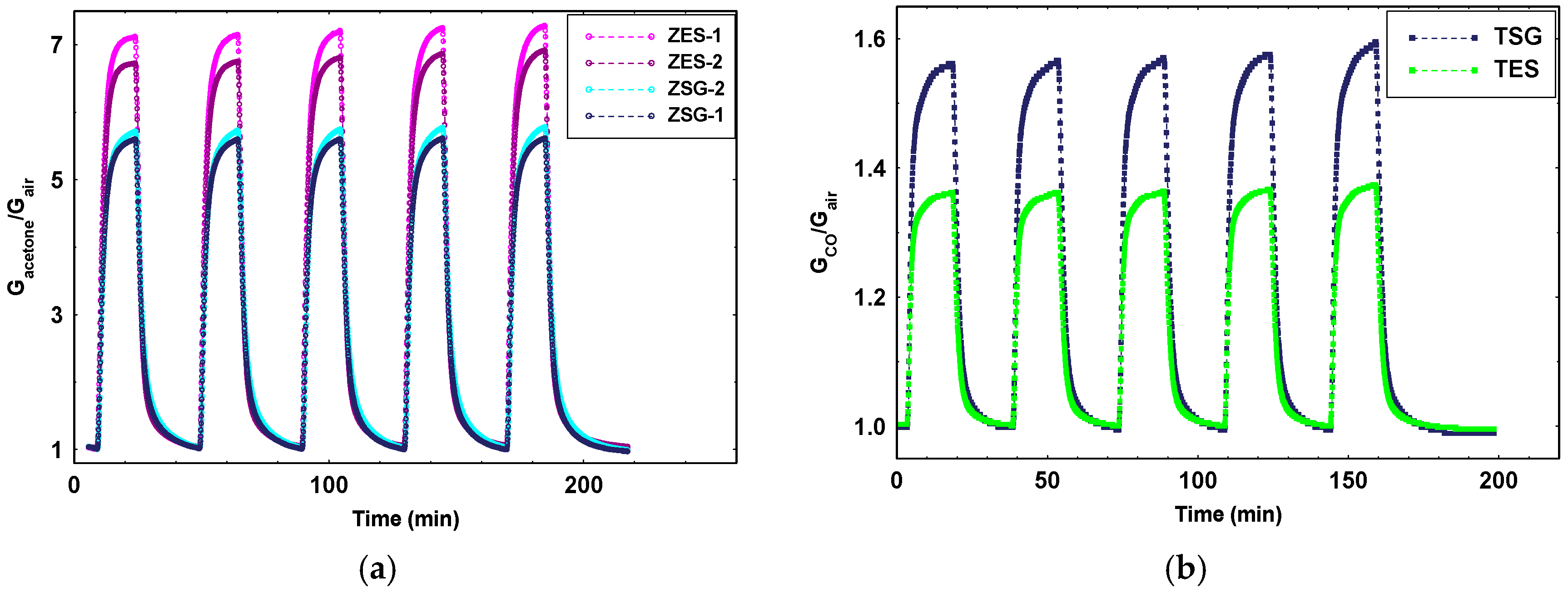
Concerning the gas sensing properties, at first, all ZnO films have been tested toward acetone, acetaldehyde, isoprene, ethanol and ammonia (10 ppm in dry air).
Dynamic responses were achieved with the operating temperature ranging from 350 to 550 °C. It resulted that all investigated samples exhibited the best performances towards acetone, giving the other gases weak interference with respect to it, except isoprene and ethanol for which the response was about one third with respect to acetone. In
Figure 4, a series of responses to 1 ppm of acetone in dry air at the working temperature of 400 °C for ZES samples and 450 °C for ZSG ones are reported. ZES samples show the best performance with respect to ZSG ones. This fact is expected due to the smaller grain size of ES-ZnO material and confirmed by IR analysis. Moreover, measurements of intergranular energy barrier versus temperature (not shown here), exhibited a greater difference between the minimum and the maximum of the barrier, being 1.1 eV for ZES samples and 0.6 for ZSG ones. It means that in ZES samples many more ionosorbed oxygens than in ZSG are available for surface chemical reactions causing a greater response to gases. Moreover, other measurements (not reported here) showed the ability of this material to detect acetone at concentrations low down to tens of ppb.
Passing to TiO2 films, they were tested toward carbon monoxide (10–1 ppm), benzene (2 ppm), hydrogen sulfide (5 ppm) and sulphur dioxide (5 ppm). Both synthesized materials have been able to detect all the tested gases. In particular, they resulted able to detect carbon monoxide at concentrations suitable for the environmental application. However, differently from the case of ZnO, SG-TiO2 exhibited the best performances toward each tested gas, despite the very fine particles of which the fibers of the electrospun TiO2 are constituted. The lower ability of ES-TiO2 to detect very low concentration of gases could be ascribed to: (i) a great percentage of rutile phase present on the films fired at 650 °C; (ii) the elongated structure could not guarantee an adequate number of contacts between fibers to favor the formation of Schottky barriers. However, further investigations must be carried out to verify these points.