Improving Sensitivity of a Chemoresistive Hydrogen Sensor by Combining ZIF-8 and ZIF-67 Nanocrystals †
Abstract
:1. Introduction
2. Materials and Methods
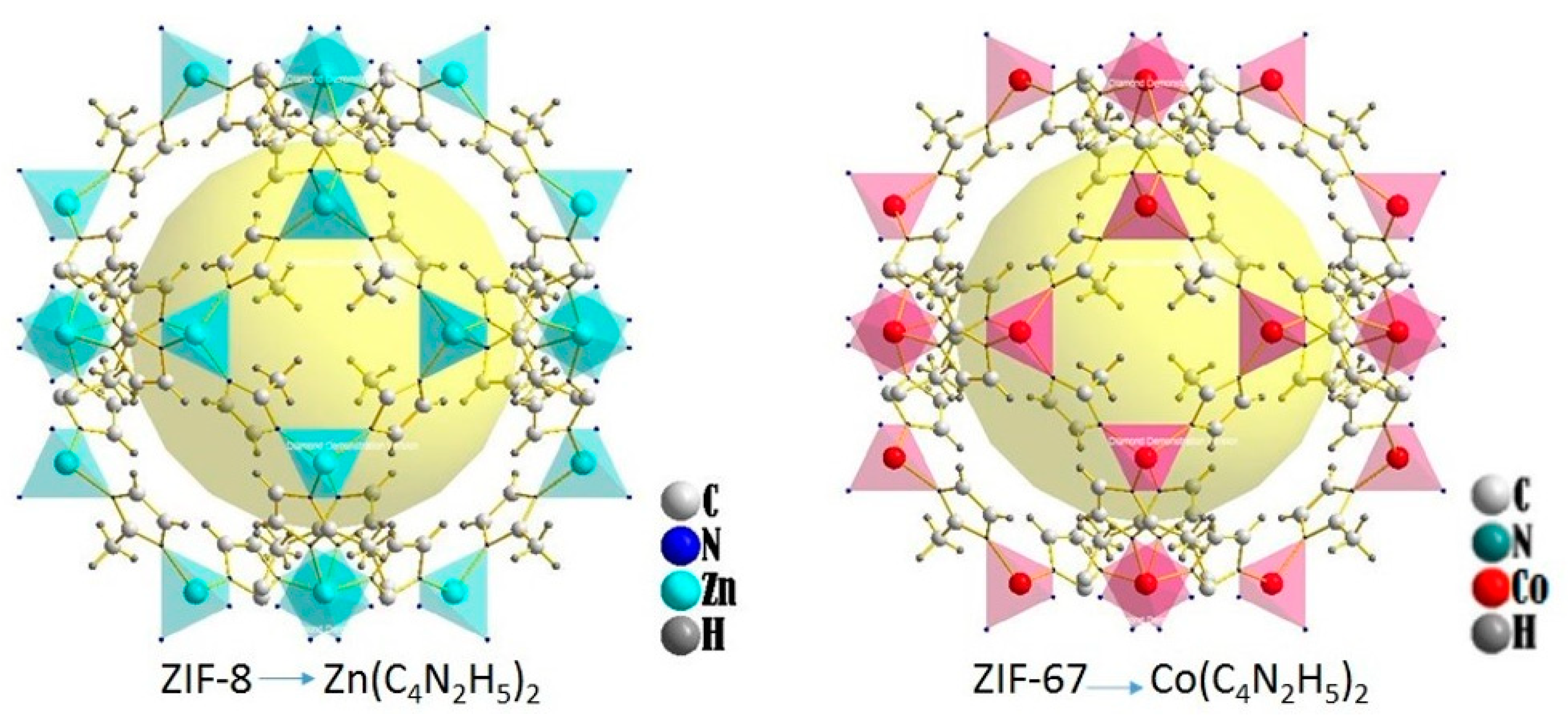
2.1. Synthesizing of ZIF-8 and ZIF-67
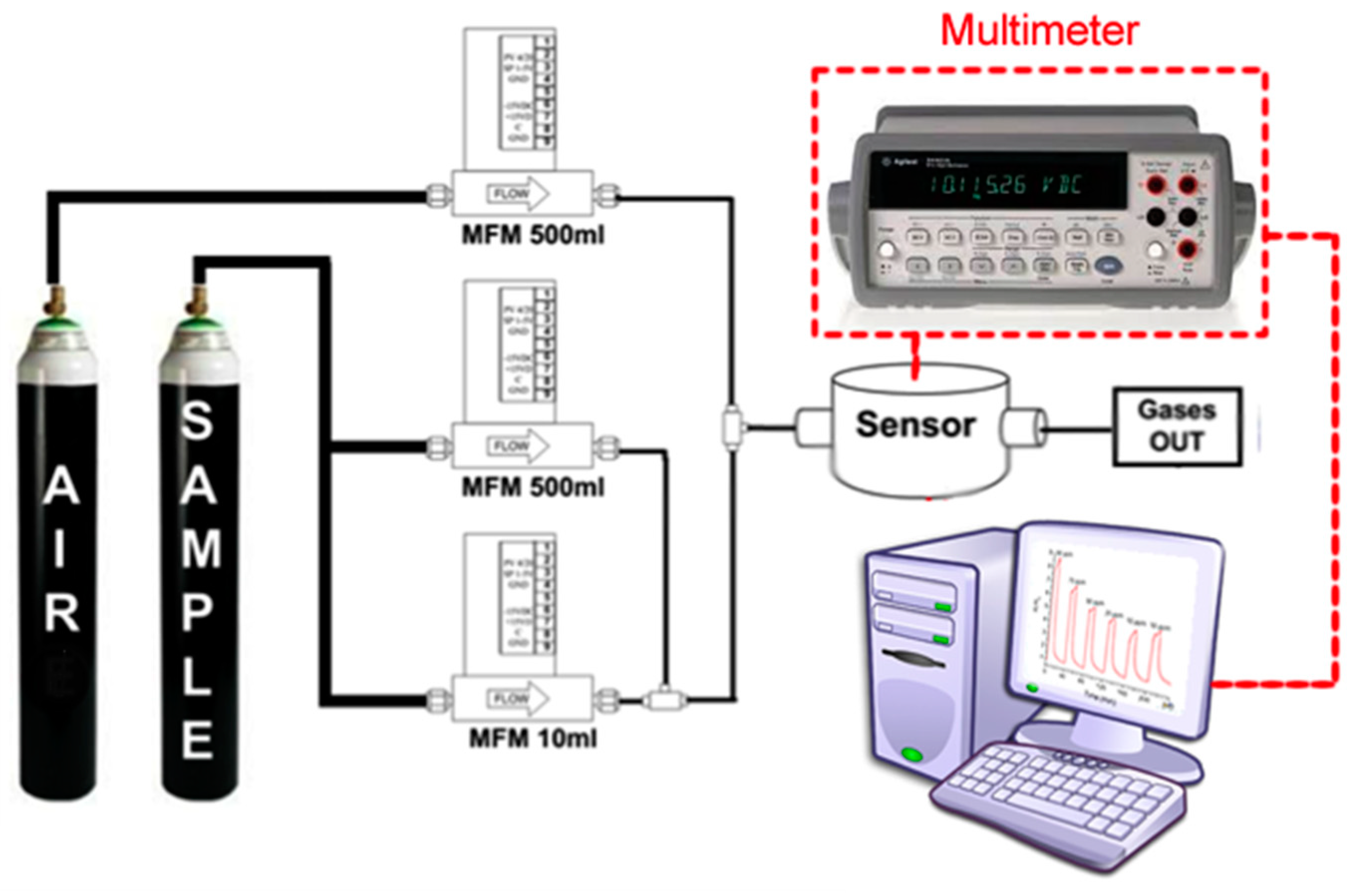
2.2. Experimental Setup
2.3. Sensor Fabrication
3. Results
3.1. Electrical ZIFs Characterization
3.2. Estructural and Morphological ZIFs Characterization

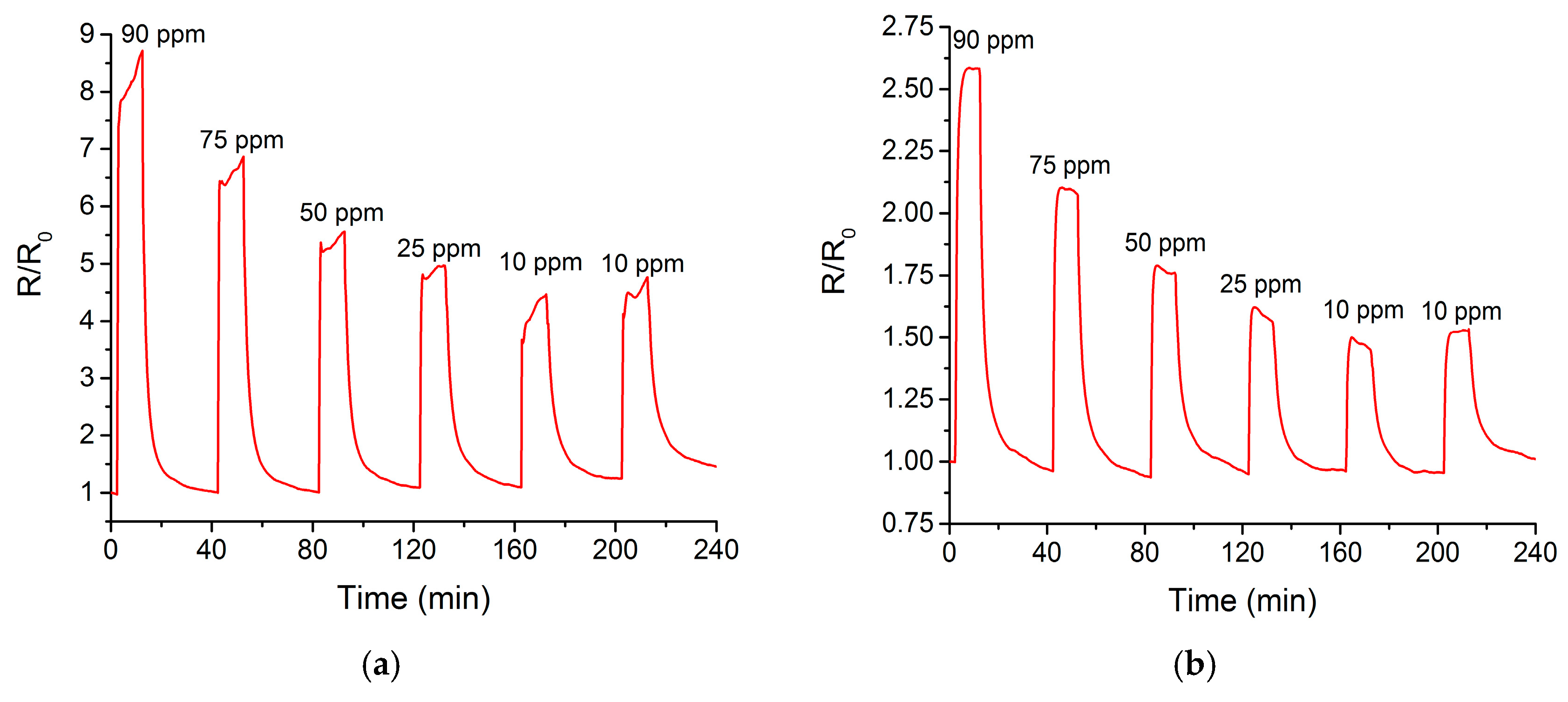
3.3. Hidrogen Sensor Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Da Silva Veras, T.; Mozer, T.S.; da Costa Rubim Messeder dos Santos, D.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Thruoghput Synthesis of Zeolite Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matatagui, D.; Sainz-Vidal, A.; Gràcia, I.; Figueras, E.; Cané, C.; Saniger, J. Improving Sensitivity of a Chemoresistive Hydrogen Sensor by Combining ZIF-8 and ZIF-67 Nanocrystals. Proceedings 2017, 1, 462. https://doi.org/10.3390/proceedings1040462
Matatagui D, Sainz-Vidal A, Gràcia I, Figueras E, Cané C, Saniger J. Improving Sensitivity of a Chemoresistive Hydrogen Sensor by Combining ZIF-8 and ZIF-67 Nanocrystals. Proceedings. 2017; 1(4):462. https://doi.org/10.3390/proceedings1040462
Chicago/Turabian StyleMatatagui, Daniel, Arianee Sainz-Vidal, Isabel Gràcia, Eduardo Figueras, Carles Cané, and José Saniger. 2017. "Improving Sensitivity of a Chemoresistive Hydrogen Sensor by Combining ZIF-8 and ZIF-67 Nanocrystals" Proceedings 1, no. 4: 462. https://doi.org/10.3390/proceedings1040462
APA StyleMatatagui, D., Sainz-Vidal, A., Gràcia, I., Figueras, E., Cané, C., & Saniger, J. (2017). Improving Sensitivity of a Chemoresistive Hydrogen Sensor by Combining ZIF-8 and ZIF-67 Nanocrystals. Proceedings, 1(4), 462. https://doi.org/10.3390/proceedings1040462




