1. Introduction
Metal oxide nanowires could be synthetized using a wide range of different techniques, each one with their own advantages and cons. Among these, evaporation-condensation techniques like vapour-liquid-solid (VLS) method are quite popular due to their low cost and high yield [
1,
2].
Tin oxide is widely recognized as the most used metal oxide semiconducting material for chemical sensing applications. In form of nanowires, n-type SnO2 is usually synthetized by VLS technique using Pt and Au as noble metal catalysts. However, it is not clear if the very small amount of nanoparticles used for the growth has an effect on chemical sensing properties. The aim of this work is to study the effect of Au and Pd nanoparticles on the performance of conductometric gas sensors compared to pure SnO2 nanowires (Sn nanoparticles).
2. Materials and Methods
In the present work SnO2 nanowires were synthetized by using VLS on alumina substrates, using different metal nanoparticles as catalyst for the growth. In particular, the effect of Au, Sn and Pd nanoparticles was exploited. The growth was performed simultaneously on all substrates, keeping the same conditions for the three different catalysts used.
Alumina substrates (Kyocera, 2 × 2 mm2, 99% purity) were cleaned in ultrasonic bath. Metal nanoparticles (Au, Sn and Pd) were deposited by RF magnetron sputtering on the top side of the samples (75 W Ar plasma, 4 × 10−3 mbar, 5 s, RT). Samples were then placed inside a tubular furnace to synthetize SnO2 nanowires. The furnace was set at 1150 °C to evaporate metal oxide powder, while samples were placed in a colder region of the furnace (620 °C) to promote the condensation of evaporated material. The pressure inside the furnace was fixed at 5 mbar, while an Argon flow of 100 sccm was used.
Nanowires were investigated by FE-SEM, HR-TEM, and XRD measurements, in order to confirm the morphology and the crystalline structure of the material.
A field-emission scanning electron microscope (FE-SEM) LEO 1525 was used to investigate the morphology of samples. Electron beam was set at 3–5 keV energy range, and samples were attached to metallic stub by carbon glue, to reduce charging effect due to the interaction of electron beam with the specimens. HR-TEM investigations were performed using a JEOL JEM ARM 200F microscope in Bucharest, at 200 kV acceleration voltage.
The XRD technique (Empyrean diffractometer, PANalytical, Netherlands) was used to investigate the crystalline properties of SnO2 nanowires. The XRD was performed in glancing angle mode, with 1.5° incident angle using Cu-LFF (λ = 1.5406 A°) operated at 40 kV to 40 mA. Spectra were recorded by a parallel-plate collimated proportional Xe detector with a nickel large-β filter, in the range of 10–90 degrees.
Finally, conductometric sensing devices were fabricated depositing electrodes on top of these materials, and a heating element on the bottom faces of the samples, by magnetron sputtering. For both electrodes and heater, soldering pads were firstly deposited by DC magnetron sputtering (70 W argon plasma, 300 °C, pressure 5.3 = 10−3 mbar), using a titanium tungsten alloy as an adhesion layer. Afterwards, interdigitated platinum contacts and heating element were deposited in order to have high electrical conductance with stable gold wire bonding.
Chemical sensing performances were evaluated toward some typical air pollutants, in particular CO and Acetone, a typical volatile organic compound (VOC). Different concentrations of these two gases were introduced in our test chamber and the temperature of each sensing device was controlled, in order to identify the optimal one. The response was calculated as the variation of the electrical conductance upon the baseline (∆G/G).
3. Results
The morphology of nanowires was investigated prior to structural analysis.
Figure 1 reports FE-SEM pictures of synthetized materials. nanowires morphology is not influenced by metal catalyst used. For all three catalyst investigated, the aspect ratio of SnO
2 nanowires is very high, with a typical length of some micrometers compared to the average diameter of few tens of nanometers. The length of nanowires can be easily tuned increasing or decreasing deposition time.
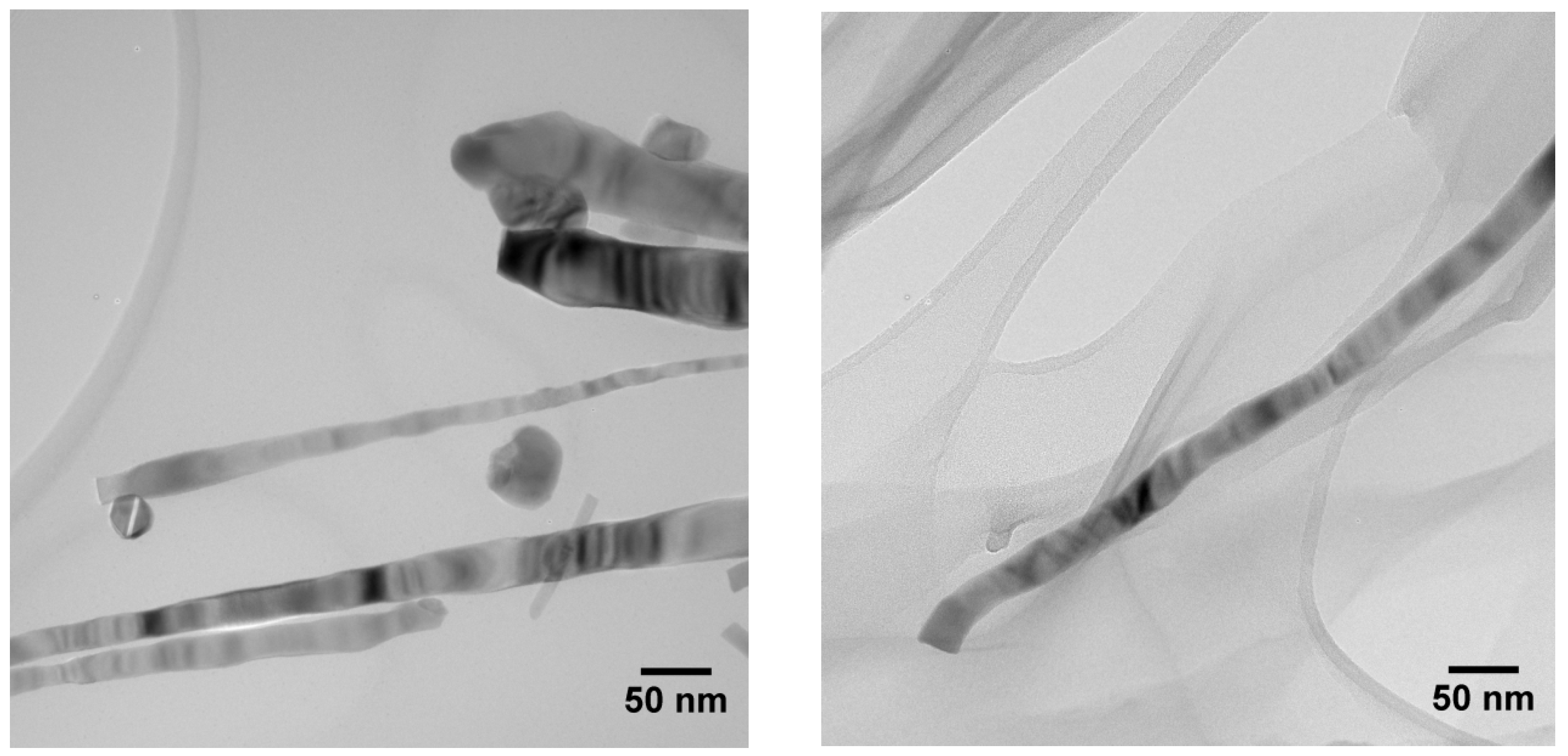
HR-TEM investigations confirm the crystalline nature of SnO
2 nanowires (
Figure 2). More specifically, in
Figure 2 left are clearly visible the gold nanoparticles promoting the growth of the nanowires. These nanoparticles are not integrated inside the tin oxide nanostructures, and, at the end of the deposition, they stay close to the tip of the nanowires. On the contrary, tin nanoparticles became part of the nanowires during the growth process (
Figure 2 right). XRD measurements perfectly match cassiterite crystal structure, a typical configuration for tin oxide, for all three different materials.
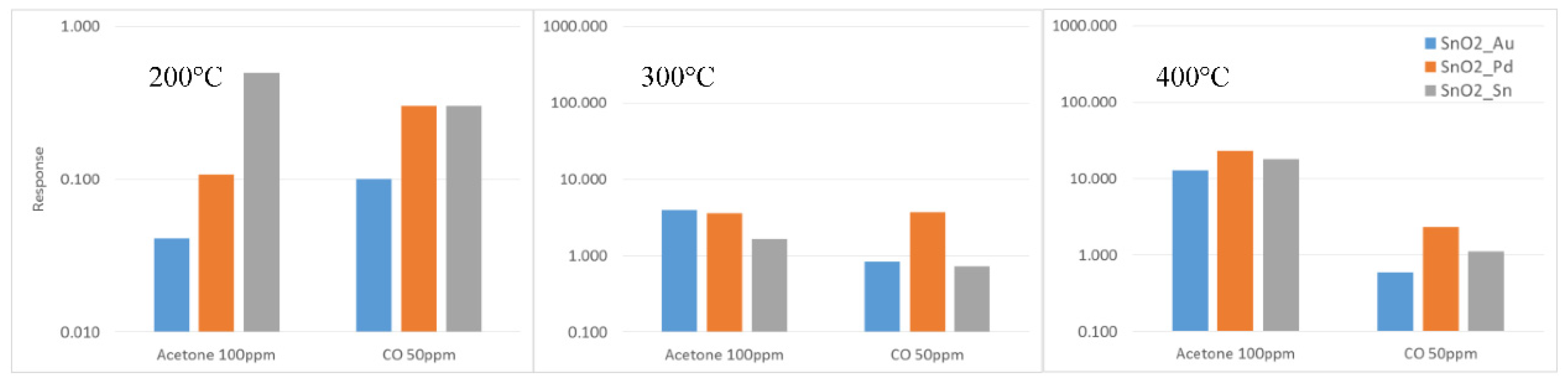
Figure 3 reports the response of Au, Pd and Sn catalyzed tin oxide nanowires towards fixed concentrations of acetone and carbon monoxide, at 200, 300 and 400 °C. Pd-catalyzed SnO
2 nanowires gave the overall highest response for both gases, especially at higher temperature. In some cases, at lower temperature, Sn catalyzed materials exhibit higher response to acetone and CO. At 200 °C gold catalyzed nanowires exhibit the lower performances.
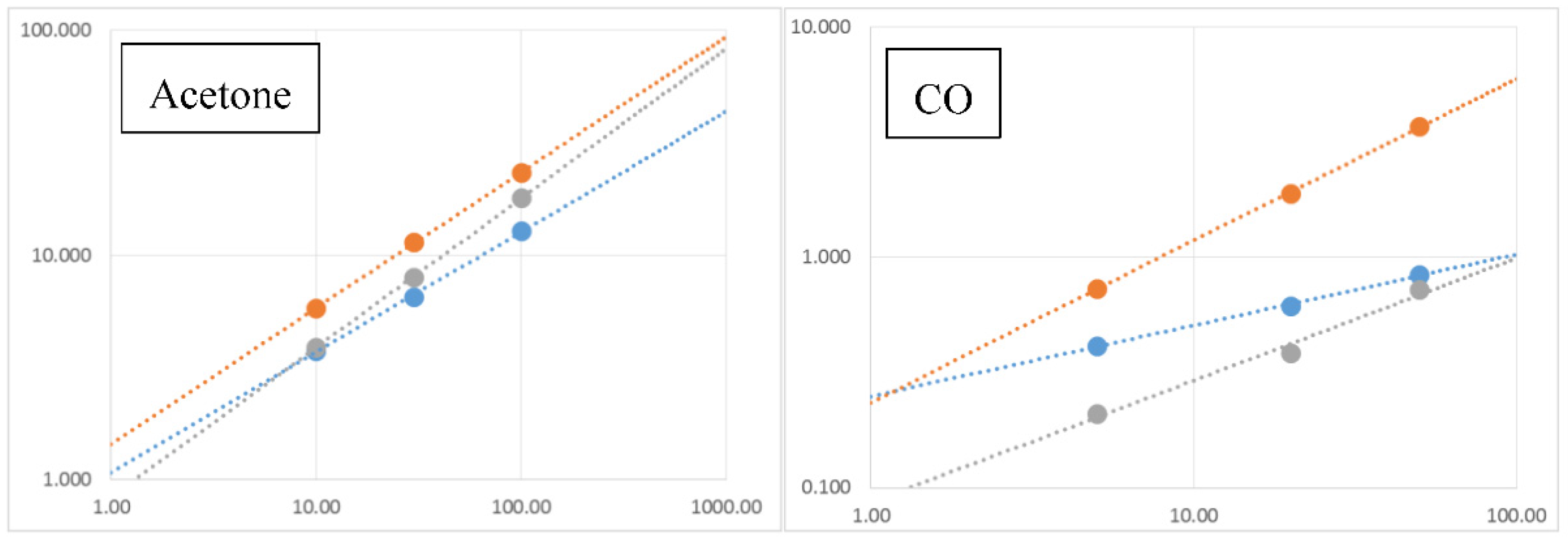
Calibration curves were calculated by using the typical power trend law for metal oxide materials, and they are reported in
Figure 4. The calibration curves are in strong agreement with the data points, and they confirm the highest performances of Pd catalyzed nanowires compared with Au and Sn ones. The detection limit, calculated as ∆G/G = 1, for Acetone is estimated at less than 1 ppm, an interesting result that allows the use of these devices for practical acetone detection.
4. Conclusions
SnO2 nanowires were synthetized by VLS technique by using three different metal catalyst (Au, Pd and Sn) under the same experimental conditions, in order to evaluate the effect of the presence of a growth catalyst on the chemical sensing performances.
The morphology and the structure of the materials have been evaluated, confirming the nanowire morphology. HR-TEM and XRD analysis confirm their high crystalline structure.
Gas sensing measurements highlight the dependence of the response with the growth catalyst used. At high temperatures, Pd catalyzed nanowires exhibit better performances than Au and Sn ones, enhancing up to five times the response compared to Sn ones.
Thanks to the different response spectra, due to the metal catalyst used, and easy fabrication, these devices are ideal candidates to be integrated into arrays, which can potentially be included into state-of-the-art electronic noses.