Abstract
Among all NOx reduction approaches, selective catalyst reduction (SCR) system is one the most reliable ways to control NOx emissions from diesel engine vehicles and trucks. In order to optimize the conversion rates of NOx and to prevent inducing excessive NH3 to the air, an NH3 and NOx sensor is required. In this study, three different types of YSZ ink have been examined to identify the most effective electrolyte for NOx and NH3 sensing. The results have shown that a home-made ink with YSZ powder shows the best sensitivity towards ammonia without polarization and the highest NO2/NO signal ratio with polarization current of 25 nA.
1. Introduction
Nowadays, one of the biggest concerns of public and politicians, is air pollution with a major focus on poor air quality and its effects on the life in urban areas [1]. In order to decrease this concern, a lot of NOx (which mostly contribute to NO2 concentrations) reduction approaches have been developed recently which among them Selective Catalytic Reduction system (SCR) is one of the most cost-effective and fuel-efficient diesel engine NOx emissions control technologies. Since the DeNOx selective catalytic reduction system uses ammonia as the reducing agent, the concern of NH3 being injected into the atmosphere has been rose up in heavy duty diesel vehicles [2]. J.P. Viricelle et al. in 2015 [3] have developed mixed-potential selective NO2 sensors. Based on this study, we aim to develop sensors for selective detection of both NOx and NH3 for automotive exhaust.
2. Materials and Methods
In this study, different sensors are fabricated by using commercial and home-made ink by the method of screen-printing. The used inks are consisting of a powder of inorganic material (active element), a binder and an organic solvent. As solid electrolyte we used a commercial YSZ ink (named ink A) sintered at 1380 °C or 1480 °C and also home-made ink using YSZ powder (named ink B) sintered at 1380 °C. For the electrodes, we used gold (ESL 8880-H) and platinum (ESL 5545) commercial inks and for the connectors we used the same ink as gold electrode. The welding areas were made from a palladium and silver commercial ink (ESL 9635BT). A schematic figure of fabricated sensors is shown in Figure 1a.
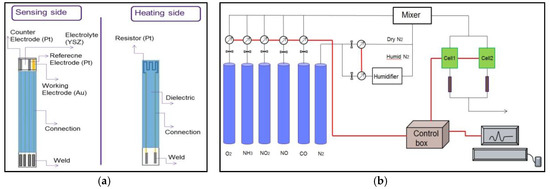
Figure 1.
A schematic figure of: (a) solid-state fabricated gas sensors; (b) experimental set-up at EMSE.
In order to evaluate the performance of the fabricated planar sensors, the response to different pollutant gases such as CO, NH3, NO2 and NO in respective amounts of 100, 20, 100 and 100 ppm are studied at 450 °C and in a base gas composed of 12% O2 and 1.5% H2O, balanced with N2. The test bench that we use in this study is presented in Figure 1b. The gases are controlled by mass flow regulators and arrive first in a mixing chamber before being sent to two cells which each contains one sensor. A vaporizer allows performing the tests in humid atmosphere.
In addition, by applying a constant polarization current of 25 nA between working electrode (Au) and counter electrode (Pt), the sensor responses (ΔV) are measured between working electrode and reference electrode (Pt) according to Equation (1).
: Potential of the reference electrode; : Potential of the working electrode.
3. Results and Discussion
In order to take into account irreproducibility of the sensors due to fabrication process, all tests are repeated with two sensors (1 and 2). The OCV values (Open Circuit Voltage, no polarization) were measured in the base gas for 30 min upon . In Figure 2, we observe the expected response in the opposite direction for reducing gas such as CO, NH3 and NO (positive comparing with the baseline) and the oxidizing gas NO2 (negative to baseline).
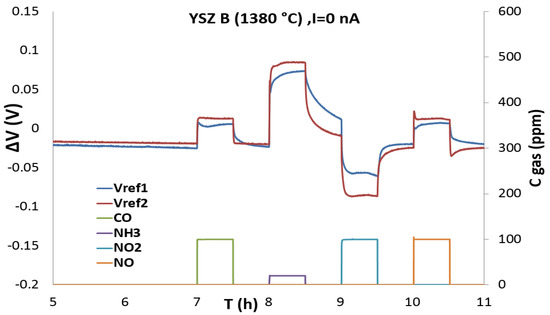
Figure 2.
Responses (ΔV) of the three-electrodes sensors with YSZ B at 450 °C during pulses of CO (100 ppm), NH3 (20 ppm), NO2 (100 ppm) and NO (100 ppm) in base gas without polarization current.
Since NO2 is an oxidizing gas, the galvanostatic mode can only produce the electro-chemical reduction of NO2 at working electrode. Figure 3 shows the variations of the potential (ΔV) for sensors with YSZ B at 450 °C for different gases by applying a polarization current of 25 nA. In these conditions it can be obviously seen that the responses to other interfering gases such as CO and NH3 are almost removed while there is relatively a good selectivity towards NO2.
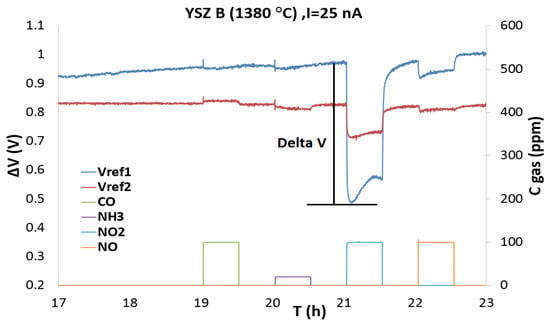
Figure 3.
Responses (ΔV) of the three-electrodes sensors with YSZ B at 450 °C during pulses of CO (100 ppm), NH3 (20 ppm), NO2 (100 ppm) and NO (100 ppm) in base gas with a polarization current of 25 nA.
The responses of different sensors (YSZ A 1380 and 1480 °C, YSZ B 1380 °C) to 20 ppm of ammonia are shown in Figure 4. This bar chart clearly illustrates that without polarization the sensors with YSZ B give the most significant responses toward NH3. Regarding NO2/NO signal ratio, although the sensors based on commercial YSZ ink A sintered at 1380 °C show the highest response to both gases, Figure 5 shows that the most relevant sensor in regards of NO2 response is the one with YSZ B.
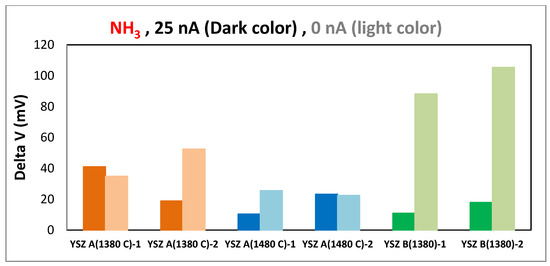
Figure 4.
The responses of different sensors to 20 ppm of ammonia at 450 °C.
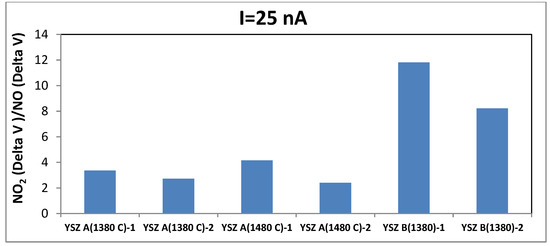
Figure 5.
NO2/NO signal ratio of different sensors at 450 °C (100ppm for each gas).
4. Conclusions
Comparing all these results, it can be concluded that sensors made with home-made ink B give more confident results regarding NOx and ammonia gases. Further investigations on chemical composition of these YSZ inks and structure of resulting electrolyte film will be performed in order to explain such different sensor responses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hitchcock, G.; Conlan, B.; Kay, D.; Brannigan, C.; Newman, D. Air Quality and Road Transport: Impacts and Solutions; RAC Foundation: London, UK, 2014. [Google Scholar]
- Suarez-Bertoa, R.; Zardini, A.A.; Astorga, C. Ammonia exhaust emissions from spark ignition vehicles over the new European driving cycle. Atmos. Environ. 2014, 97, 43–53. [Google Scholar] [CrossRef]
- Romanytsia, I.; Viricelle, J.P.; Vernoux, P.H.; Pijolat, C. Application of advanced morphology AuX (X = YSZ, ZrO2) composites as sensing electrode for solid state mixed-potential exhaust NOx sensor. Sens. Actuators B Chem. 2015, 207, 391–397. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).