1. Introduction
Elemental mercury (Hg) is extremely toxic. It is known to affect the lung, kidney, central and peripheral nervous system. According to the World Health Organization [
1], the annual average threshold exposure limit is 1 μg/m
3. In order to reduce the amount of mercury emitted into the atmosphere, there is a strong need for the development of continuous mercury detection systems which are highly sensitive, portable and durable.
The aim of this work is to investigate a novel approach for the detection of mercury vapor in air. Similar to sensors known from literature [
2], the approach is based on the amalgamation of gold. However, in this work, the novel idea is to compare the temperatures of an amalgamated and a non-exposed gold film while heating them with identical power, as it is shown in
Figure 1. The temperature difference ΔT between both films will be considered as a measure for the mercury concentration at a given exposition time. Based on theoretical considerations, providing identical heating power, the amalgamated gold film might reach higher temperatures compared to the non-exposed gold film due to the decreased heat dissipation in the environment, since the amalgamated gold surface acts like thermal insulation layer.
However, at temperatures exceeding 150 °C [
3], mercury atoms desorb from the gold film and the gold film regenerates. Since a decreasing amalgam layer thickness leads to less thermal insulation, the temperature difference also decreases for higher temperatures. Additionally, the energy required for desorption processes results in a cooling. Accordingly, depending on the heating temperature, there are two opposite effects occurring during heating the gold film. For heating temperatures below 150 °C, desorption processes are not affecting the temperature difference. Hence, a positive temperature difference is expected. However, for higher heating temperatures, desorption processes and enthalpy might lead to a decreasing temperature difference.
In the following, the actual influence of these effects on the measureable temperature difference will be investigated, considering adsorption and desorption of mercury vapor on the gold film of the sensor.
2. Sensor Characteristics
In this work, the commercially available nanocalorimeter XEN-39470b from Xensor Integration (Delfgauw, The Netherland) is used as thermal sensor for mercury vapor. The nanocalorimeter consists of two thermally isolated gold-coated hot spots with an outer diameter of 150 μm each on a thin silicon-nitride (SiN) membrane. Each hot spot can be heated by two concentrically arranged heating resistors. Due to the very low heat capacity in the nJ/K range, the hot spot reaches end temperatures up to 800 °C and cools down to room temperature within a few milliseconds. The temperature is measured by a thermocouple of n-type vs. p-type poly-silicon positioned in the center of the hot spot.
The size of the measuring effect is determined by the amount of mercury adsorbed on the gold film and the time required for desorption. Therefore, the mercury vapor concentration, which is driven out from an amalgamated gold hot spot when heating the sensor, is measured in the exhaust gas.
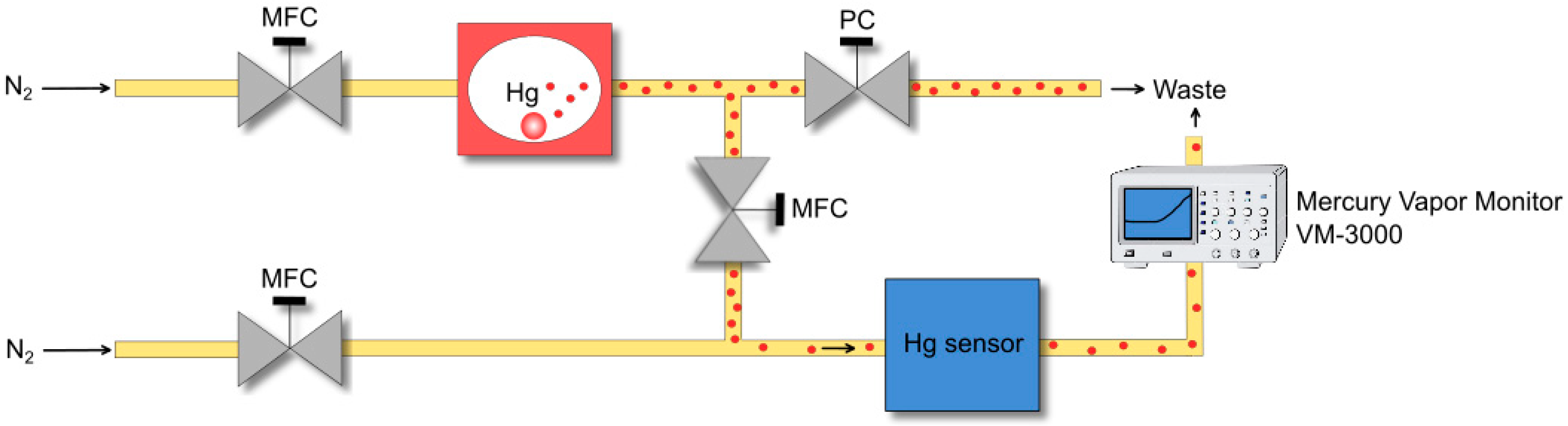
As shown in
Figure 2, the sensor is housed in a secured chamber which is continuously flows through by 50 mL/min dry, purified nitrogen. By enriching the nitrogen with mercury vapor, concentrations between 0 and 1.5 mg/m
3 can be adjusted. The exhaust gas behind the sensor chamber is analyzed by a commercially available mercury vapor monitor (VM-3000, Mercury Instruments, Littleton, CO, USA). For the investigation of the adsorption and desorption behavior, the sensor is exposed to mercury vapor for a certain time. After that, the sensor chamber is rinsed with nitrogen until the mercury concentration detected by the mercury vapor monitor in the exhaust gas is zero. Then, the gold film of the sensor is heated and the mercury vapor concentration driven out from the gold film is measured in the exhaust gas. In
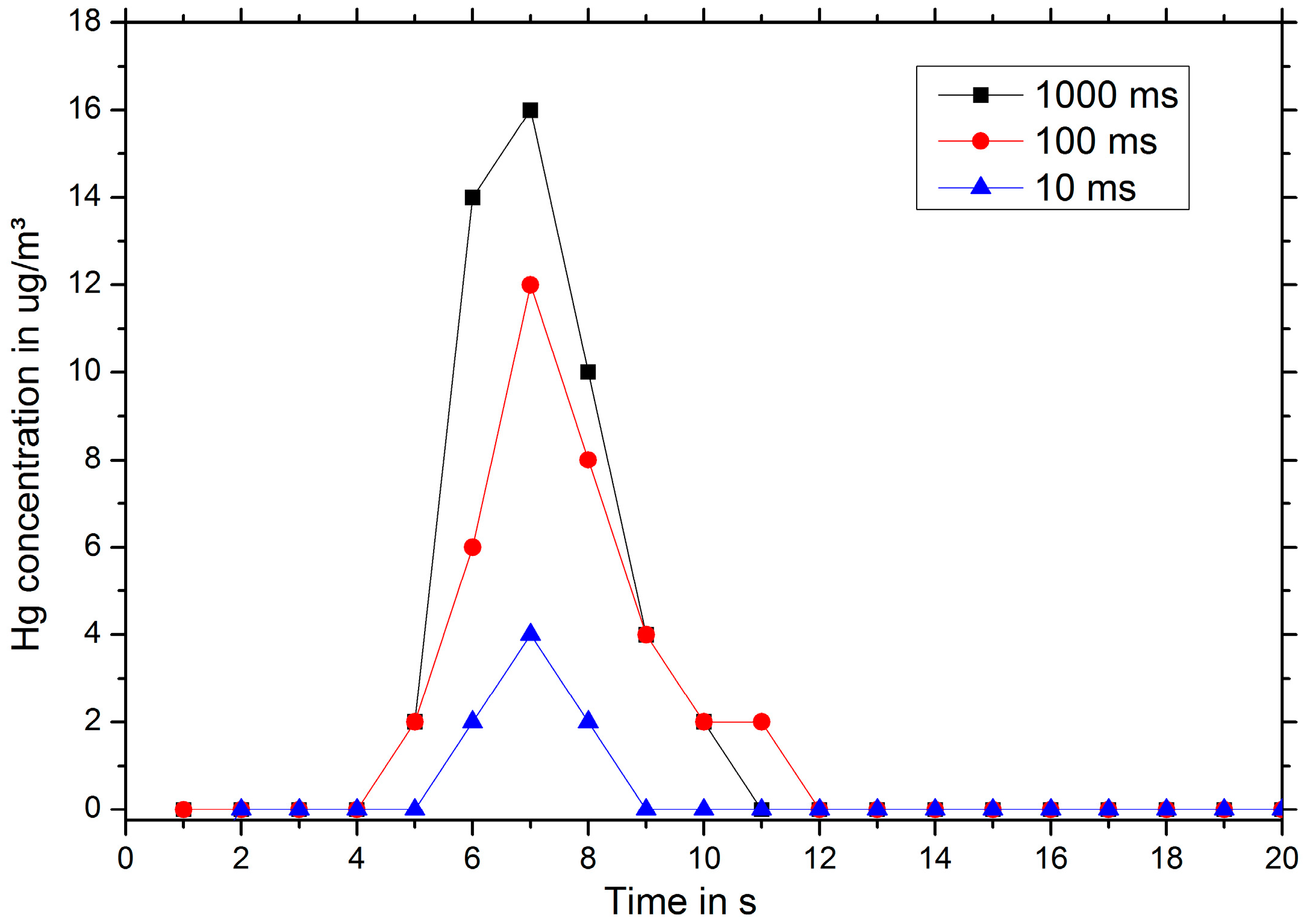
Figure 3, the measured mercury vapor concentration in the exhaust gas is plotted over time for a sensor being exposed to 1.5 mg/m
3 mercury vapor for 30 min and then heated at
t = 0 s to 800 °C for 1 s, 100 ms and 10 ms.
Although the gold film is heated only for one second, mercury vapor is detected in the exhaust gas over a period of several seconds. This is due to the diffusive broadening of the mercury plug during the transport to the mercury vapor monitor. Decreasing the heating time, the total amount of mercury detected decreases as a result of incomplete desorption (see
Figure 3).
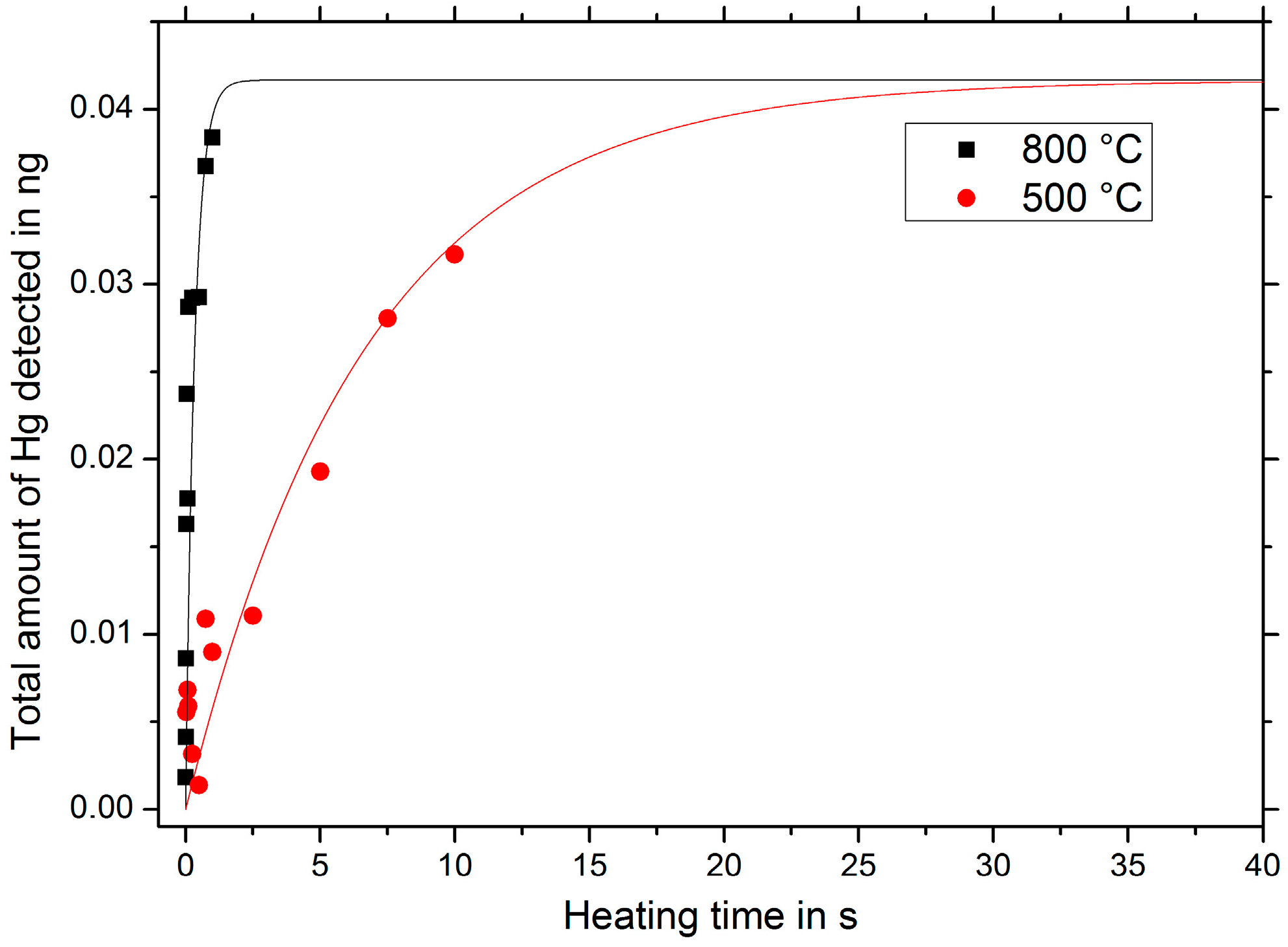
Varying the heating time in the range of 0 to 10 s, it can be seen in
Figure 4, that the desorption process is completed within approximately one second for a heating temperature of 800 °C. Using a heating temperature of 500 °C, the desorption process takes up to 20 s.
In order to estimate the influence of the desorption enthalpy on the temperature of the gold film, the desorption energy required per time is calculated in Equation (1).
with the total amount of mercury detected
, the molar mass
, the heat of adsorption
[
3] and the desorption time
. For the example given in
Figure 3 (exposition time of 30 min, mercury vapor concentration of 1.5 mg/m
3, heating temperature of 800 °C), Equation (1) delivers 3 nW. However, in the literature, for ultra-sensitive calorimetric devices detection limits around 1 μW have been reported [
4]. The value estimated in Equation (1) for the mercury desorption is orders of magnitudes below this value. Hence, the influence of the desorption enthalpy on the temperature can be neglected and the temperature of the amalgamated gold film is determined by heat dissipation effects.
3. Results
The nanocalorimeter XEN-39470b consists of two thermally isolated gold hot spots. One hot spot is utilized as sample side and one hot spot as reference side. The sequence of measurement is chosen as follows: At first, the nanocalorimeter is exposed to a defined mercury vapor concentration for a certain exposure time. After that, only the reference side is heated to 500 °C for 60 s in order to regenerate the reference gold film. Subsequently, the differential measurement is performed. That is, sample and reference side are heated to the same temperature for 60 s and the differential temperature between sample side and reference side is measured as a function of time. In the last step, sample side and reference side are regenerated by heating both to 500 °C for 60 s.
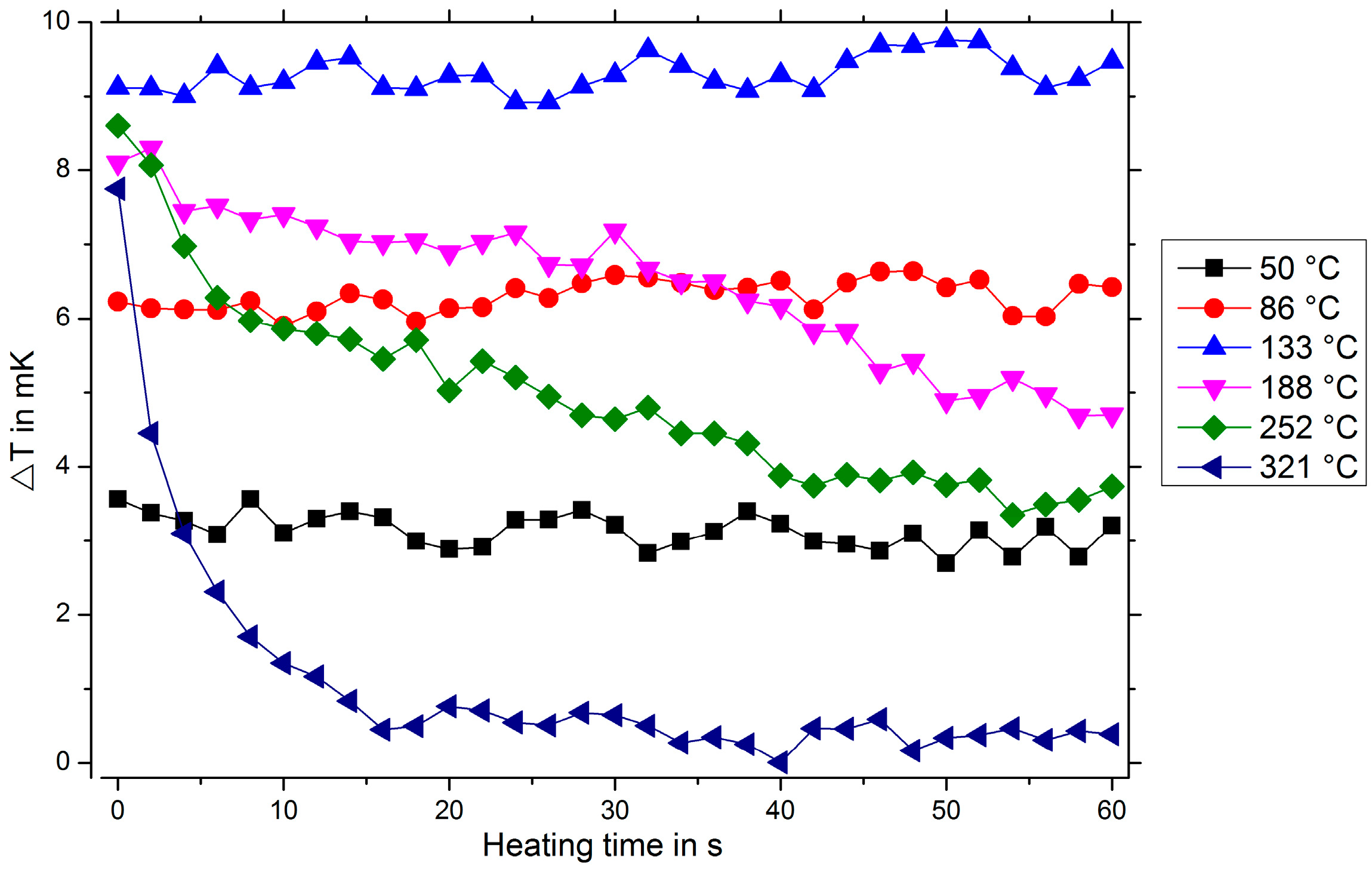
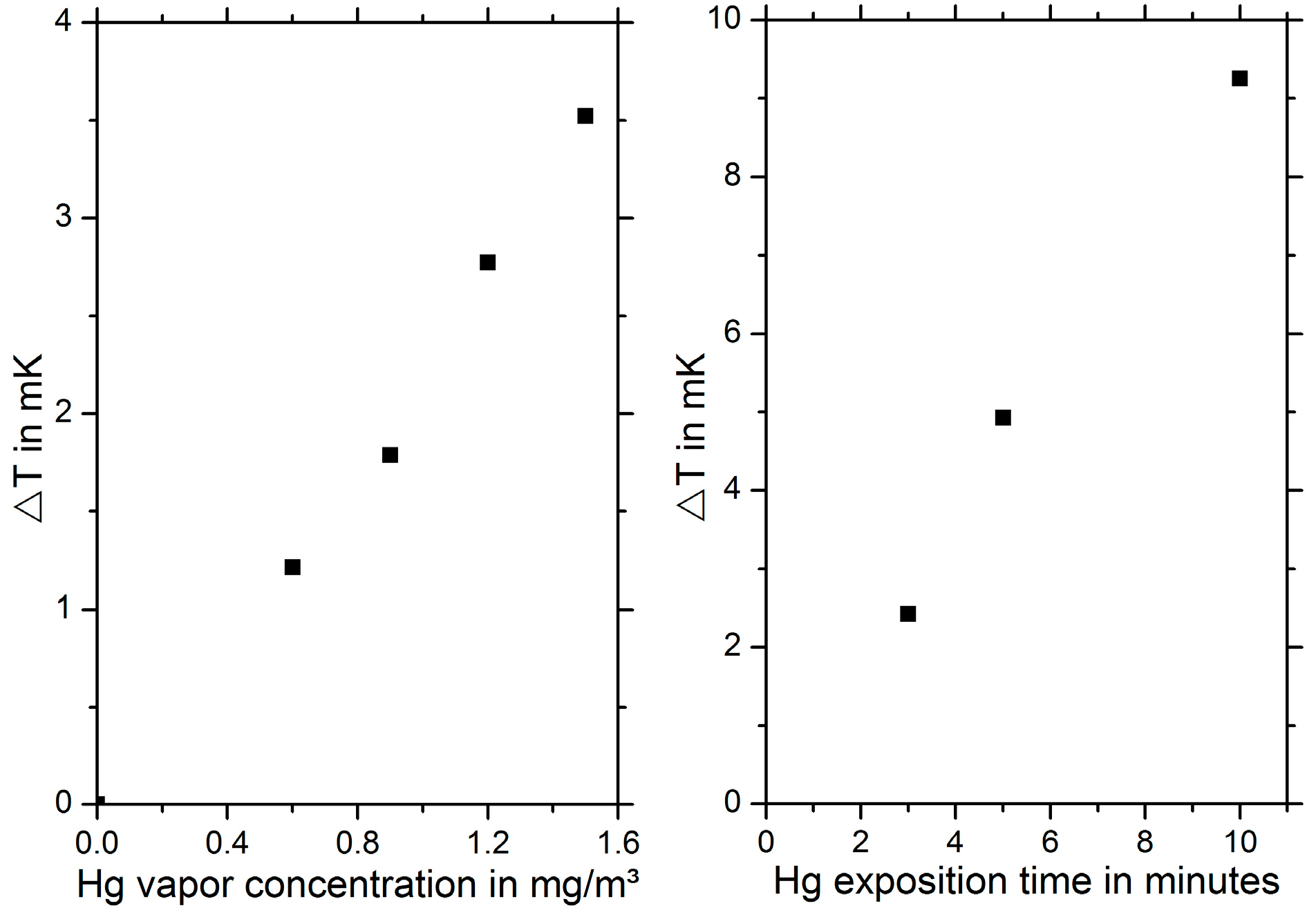
In
Figure 5, the measured temperature difference ΔT for different heating temperatures is shown. For low temperatures, ΔT increases with increasing heating temperatures. For temperatures exceeding 150 °C, a decrease in ΔT is observed due to the beginning mercury desorption, since a decreasing amalgam layer thickness leads to less thermal insulation. Furthermore, a clear dependence of the measured temperature difference on the mercury vapor concentration and the exposition time can be demonstrated, see
Figure 6.
These preliminary measurements show that a detection of mercury vapor is principally possible using this thermal approach. However, in the current setup, the signal-to-noise ratio is very low. The detection limit of our preliminary setup is 200 μg/m3 for a heating temperature of 133 °C. Thus, further investigations concentrate on lowering the detection limit. One possible approach is to improve the heating power control circuit. Furthermore, these measurements have been performed in extremely dry (<1 ppmv water content), purified nitrogen which has been enriched with mercury vapor. Other gas species also adsorb on the gold surface and influence the heat dissipation in the environment. Hence, further measurements are required investigating the influence of interfering gas species on the measured temperature difference.
4. Conclusions
In this work, we presented a novel approach for the detection of mercury vapor in air using differential heat dissipation measurements. Therefore, the temperature difference between a non-exposed and an amalgamated gold film is recorded, while heating both films with identical electrical power. As the amalgam layer lowers the heat dissipation, the amalgamated gold film reaches higher end temperatures compared to the non-exposed gold film. Preliminary experiments showed a clear dependence of the measured temperature difference on the mercury vapor concentration and the exposition time. The detection limit of our preliminary setup is 200 μg/m3. Hence, further investigations concentrate on lowering the detection limit.