Impedimetric Biosensor to Enable Fast Evaluation of Gaseous Sterilization Processes †
Abstract
:1. Introduction
2. Materials and Methods
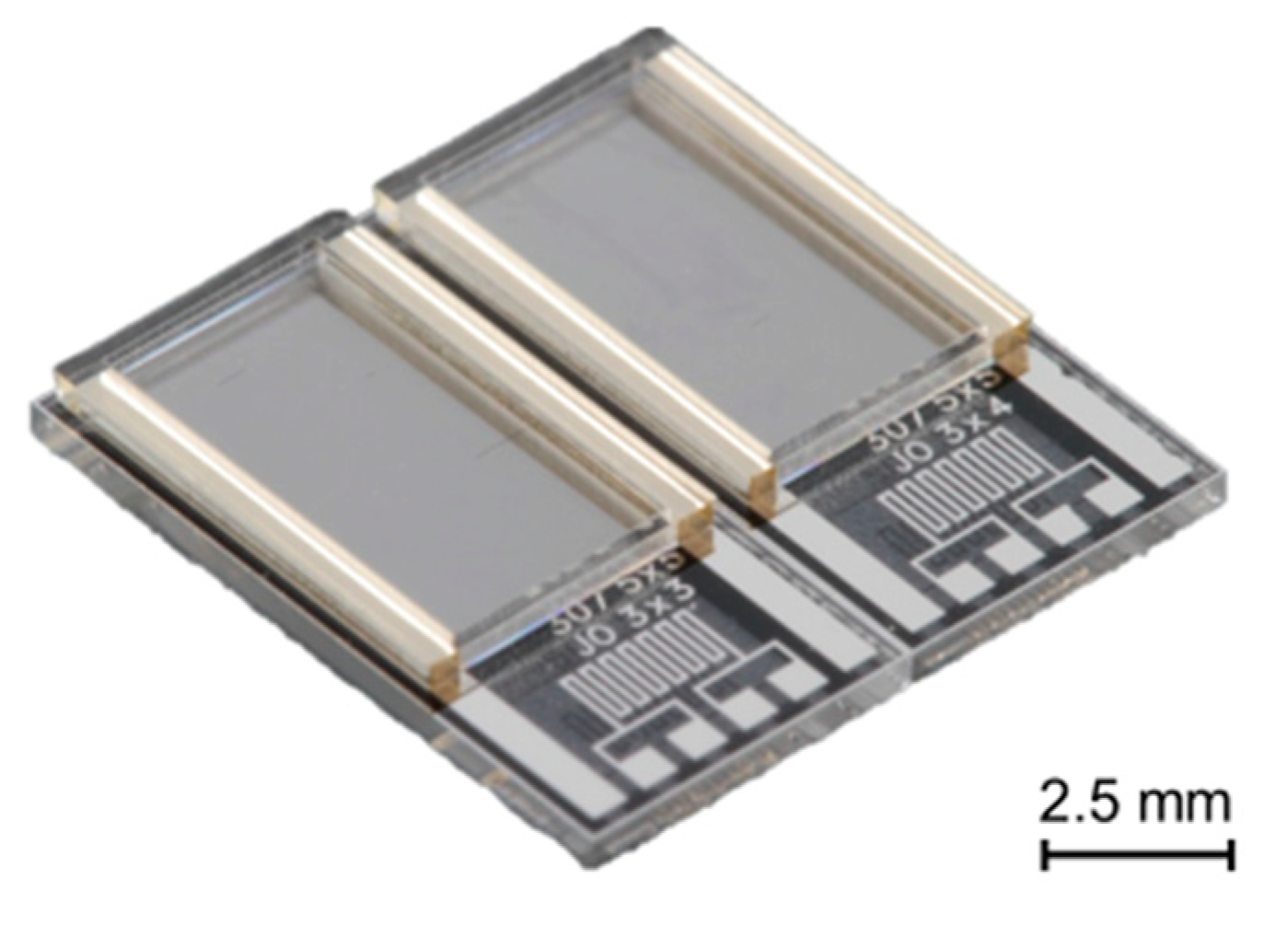
2.1. Sensor Fabrication
2.2. Impedimetric Characterization
2.3. Sterilization Process
2.4. Surface Characterisation
3. Results and Discussion
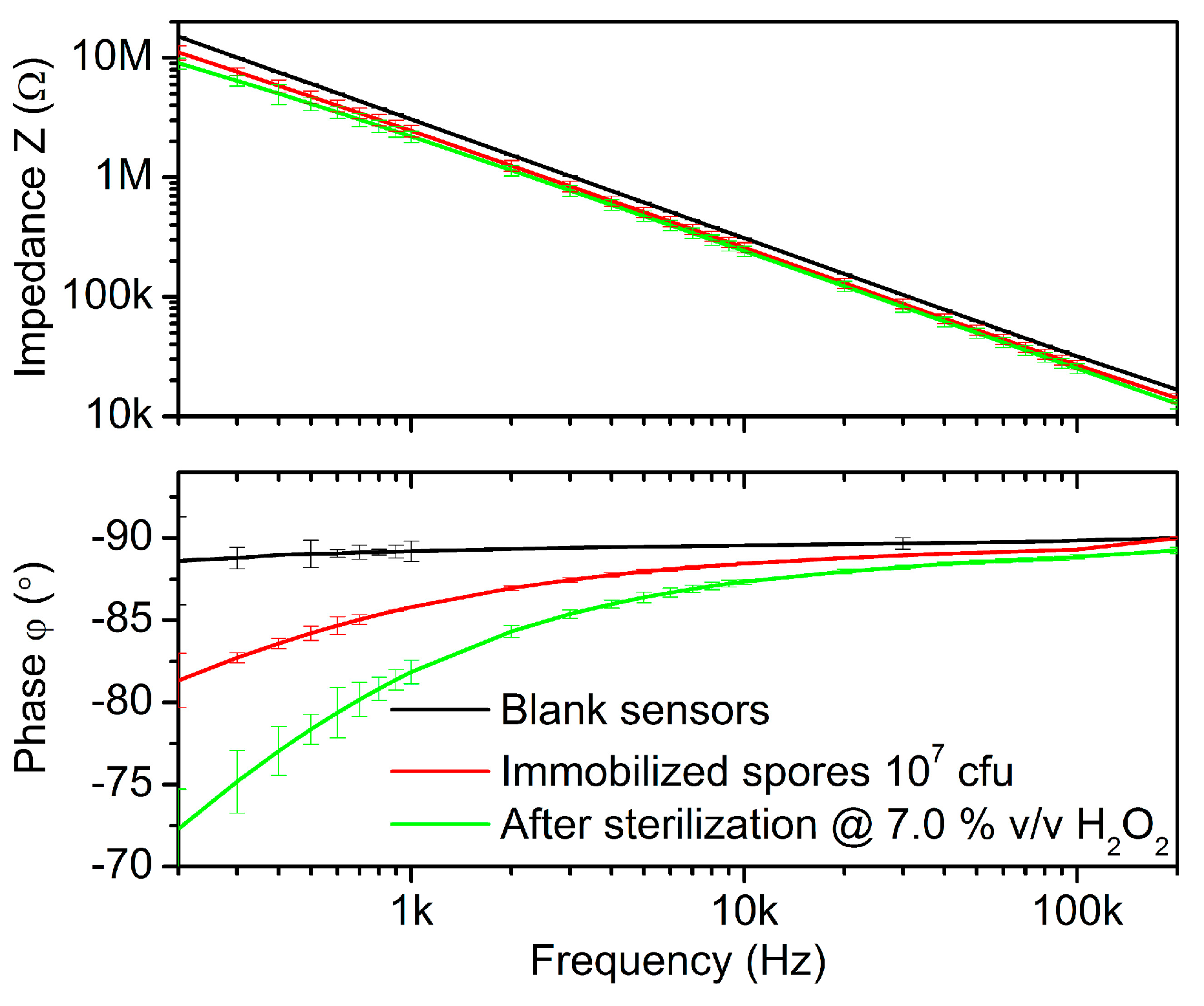
3.1. Impedimetric Results
3.2. Morphological Characterizations
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Toledo, R.T.; Escher, F.E.; Ayres, J.C. Sporicidal properties of hydrogen peroxide against food spoilage organisms. Appl. Microbiol. 1973, 26, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Ansari, I.A.; Datta, A.K. An overview of sterilization methods for packaging materials used in aseptic packaging systems. Food Bioprod. Process. 2003, 81, 57–65. [Google Scholar] [CrossRef]
- Oberländer, J.; Jildeh, Z.B.; Kirchner, P.; Wendeler, L.; Bromm, A.; Iken, H.; Wagner, P.; Keusgen, M.; Schöning, M.J. Study of interdigitated electrode arrays using experiments and finite element models for the evaluation of sterilization processes. Sensors 2015, 15, 26115–26127. [Google Scholar] [CrossRef] [PubMed]
- Oberländer, J.; Bromm, A.; Wendeler, L.; Iken, H.; Durán, M.P.; Greeff, A.; Kirchner, P.; Keusgen, M.; Schöning, M.J. Towards a biosensor to monitor the sterilization efficiency of aseptic filling machines. Phys. Status Solidi A 2015, 212, 1299–1305. [Google Scholar] [CrossRef]
- VDMA-Fachverbandsschriften. Code of practice: Filling machines of VDMA hygiene class V: Testing the effectiveness of packaging sterilization devices. VDMA-Fachverb. 2008, 6, 1–16. [Google Scholar]
- Kirchner, P.; Ng, Y.A.; Spelthahn, H.; Schneider, A.; Henkel, H.; Friedrich, P.; Kolstad, J.; Berger, J.; Keusgen, M.; Schöning, M.J. Gas sensor investigation based on a catalytically activated thin-film thermopile for H2O2 detection. Phys. Status Solidi A 2010, 207, 787–792. [Google Scholar] [CrossRef]
- Deng, X.; Shi, J.; Kong, M.G. Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasmas. IEEE Trans. Plasma Sci. 2006, 34, 1310–1316. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oberländer, J.; Arreola, J.; Hansen, C.; Greeff, A.; Mayer, M.; Keusgen, M.; Schöning, M.J. Impedimetric Biosensor to Enable Fast Evaluation of Gaseous Sterilization Processes. Proceedings 2017, 1, 435. https://doi.org/10.3390/proceedings1040435
Oberländer J, Arreola J, Hansen C, Greeff A, Mayer M, Keusgen M, Schöning MJ. Impedimetric Biosensor to Enable Fast Evaluation of Gaseous Sterilization Processes. Proceedings. 2017; 1(4):435. https://doi.org/10.3390/proceedings1040435
Chicago/Turabian StyleOberländer, Jan, Julio Arreola, Christina Hansen, Anton Greeff, Marlena Mayer, Michael Keusgen, and Michael J. Schöning. 2017. "Impedimetric Biosensor to Enable Fast Evaluation of Gaseous Sterilization Processes" Proceedings 1, no. 4: 435. https://doi.org/10.3390/proceedings1040435
APA StyleOberländer, J., Arreola, J., Hansen, C., Greeff, A., Mayer, M., Keusgen, M., & Schöning, M. J. (2017). Impedimetric Biosensor to Enable Fast Evaluation of Gaseous Sterilization Processes. Proceedings, 1(4), 435. https://doi.org/10.3390/proceedings1040435






