Picomolar Detection of Heavy Ions with Surface Acoustic Wave Sensors Functionalized with New Synthetized Anthracene Derivates †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Gravimetric Measurements
2.3. DFT Calculations
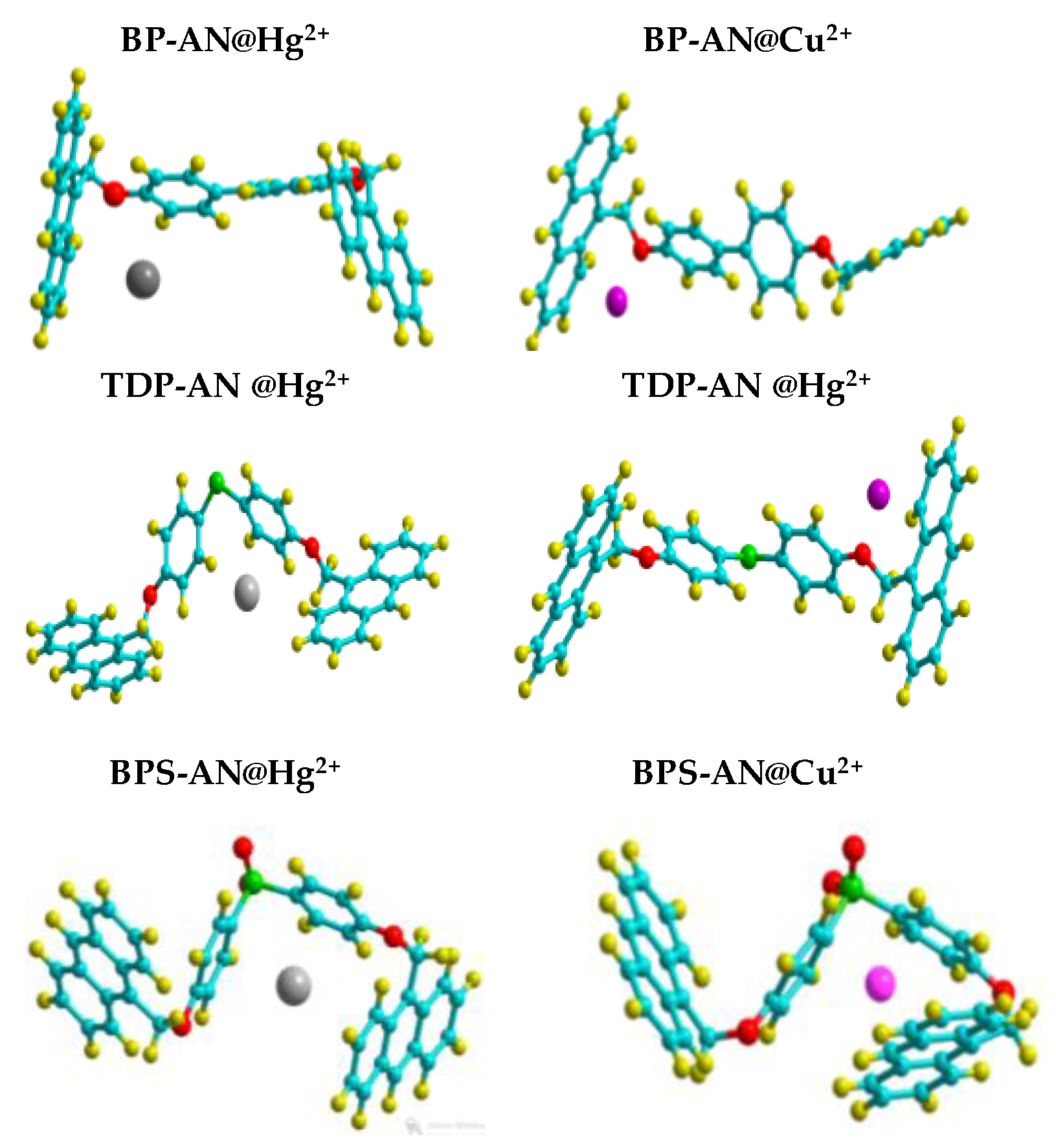
3. Results and Discussion
3.1. Gravimetric Results
3.2. Chemical Calculations
Conflicts of Interest
References
- Xu, W.; Tian, I.; Luo, Y.; Zhu, L.; Huanga, K. A rapid and visual turn-off sensor for detecting copper (II) ion based on DNAzyme coupled with HCR-based HRP concatemers. Sci. Rep. 2017, 7, 4336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xue, C.; Nan, T.; Tan, G.; Li, Z.; Li, Q.X.; Zhang, Q.; Wang, B. Detection of copper ions using microcantilever immunosensors and enzyme-linked immunosorbent assay. Anal. Chim. Acta 2010, 31, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Huang, S.; Xiong, X.; Lai, X. Synthesis and characterization of Hg(II)-ion-imprinted polymer and its application for the determination of mercury in water samples. RSC Adv. 2015, 5, 67365–67371. [Google Scholar] [CrossRef]
- Ktari, N.; Fourati, N.; Zerrouki, C.; Ruan, M.; Seydou, M.; Barbaut, F.; Nal, F.; Yaakoubi, N.; Chehimi, M.; Kalfat, R. Design of a polypyrrole MIP-SAW sensor for selective detection of flumequine in aqueous media. Correlation between experimental results and DFT calculations. RSC Adv. 2015, 5, 88666–88674. [Google Scholar] [CrossRef]
- Fourati, N.; Seydou, M.; Zerrouki, C.; Singh, A.; Samanta, S.; Maurel, F.; Aswal, D.K.; Chehimi, M. Ultrasensitive and Selective Detection of Dopamine Using Cobalt-Phthalocyanine Nanopillar-Based Surface Acoustic Wave Sensor. ACS Appl. Mater. Interfaces 2014, 6, 22378–22386. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J. Gassian09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
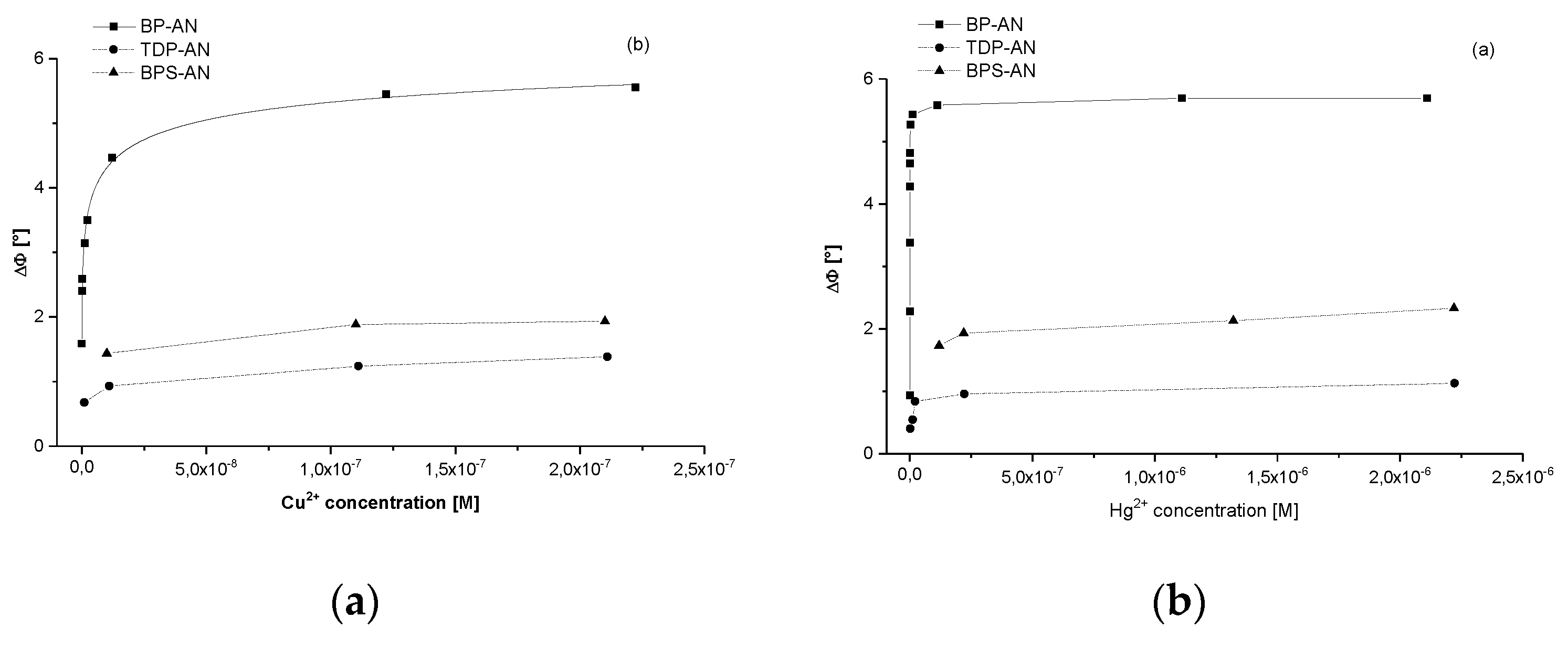
| S (°/M) | LOD (M) | |||
|---|---|---|---|---|
| Cu2+ | Hg2+ | Cu2+ | Hg2+ | |
| BP-AN | 2.24 × 108 | 3.67 × 108 | 10−12 | 10−12 |
| TDP-AN | 0.51 × 106 | 2.34 × 106 | 10−9 | 2 10−9 |
| BPS-AN | 5.8 × 105 | 2.3 × 105 | 10−8 | 10−8 |
| Eint (kJ/mol) | ||
|---|---|---|
| Cu2+ | Hg2+ | |
| BP-AN | −1370.28 | −1102.71 |
| TDP-AN | −991.51 | −923.94 |
| BPS-AN | −939.05 | −882.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, G.; Teka, S.; Rahali, S.; Fourati, N.; Zerrouki, C.; Seydou, M.; Yaakoubi, N.; Chehimi, S.; Chaabane, R.B. Picomolar Detection of Heavy Ions with Surface Acoustic Wave Sensors Functionalized with New Synthetized Anthracene Derivates. Proceedings 2017, 1, 433. https://doi.org/10.3390/proceedings1040433
Attia G, Teka S, Rahali S, Fourati N, Zerrouki C, Seydou M, Yaakoubi N, Chehimi S, Chaabane RB. Picomolar Detection of Heavy Ions with Surface Acoustic Wave Sensors Functionalized with New Synthetized Anthracene Derivates. Proceedings. 2017; 1(4):433. https://doi.org/10.3390/proceedings1040433
Chicago/Turabian StyleAttia, Ghada, Safa Teka, Saif Rahali, Najla Fourati, Chouki Zerrouki, Mahamadou Seydou, Nourdin Yaakoubi, Selim Chehimi, and Rafik Ben Chaabane. 2017. "Picomolar Detection of Heavy Ions with Surface Acoustic Wave Sensors Functionalized with New Synthetized Anthracene Derivates" Proceedings 1, no. 4: 433. https://doi.org/10.3390/proceedings1040433
APA StyleAttia, G., Teka, S., Rahali, S., Fourati, N., Zerrouki, C., Seydou, M., Yaakoubi, N., Chehimi, S., & Chaabane, R. B. (2017). Picomolar Detection of Heavy Ions with Surface Acoustic Wave Sensors Functionalized with New Synthetized Anthracene Derivates. Proceedings, 1(4), 433. https://doi.org/10.3390/proceedings1040433







