Transdermal Alcohol Measurements Using MOX Sensors in Clinical Trials †
Abstract
:1. Introduction
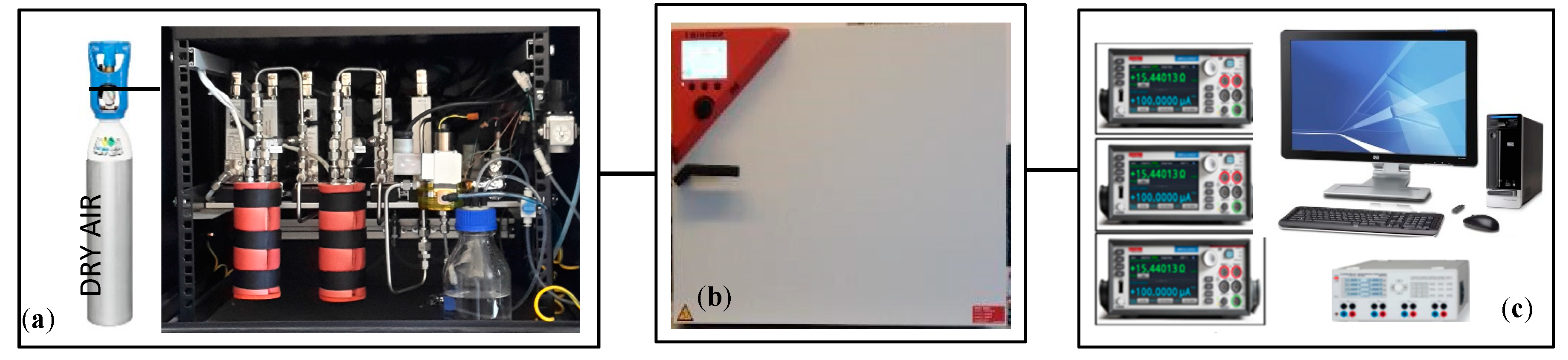
2. Experimental Set Up
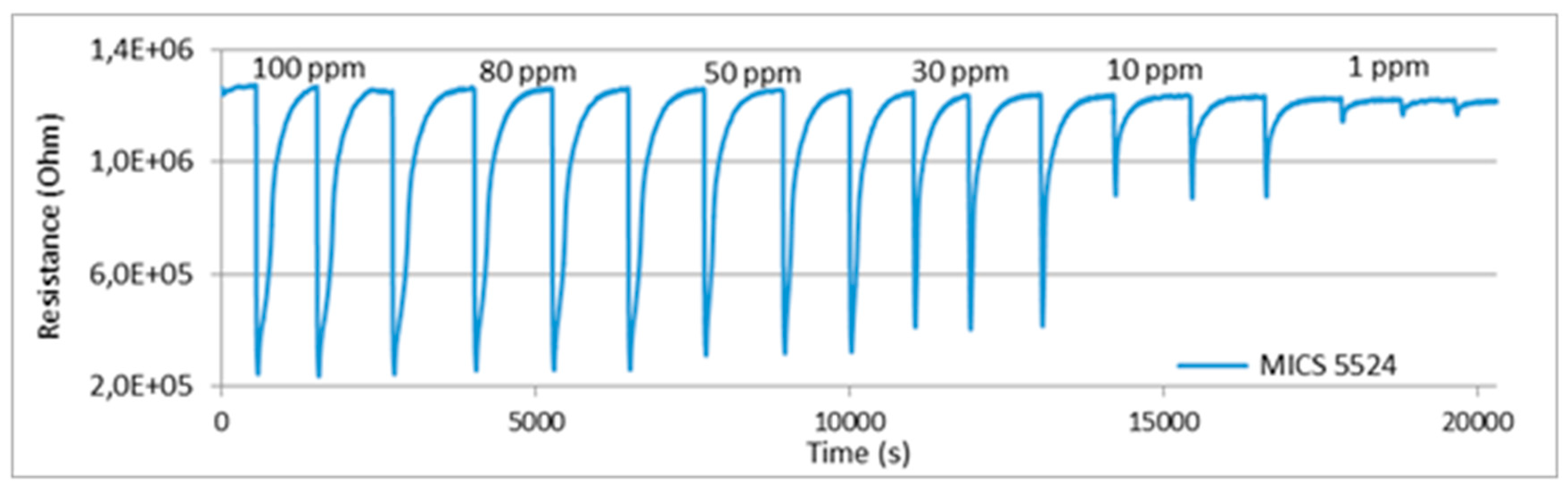
3. Sensors Calibration in the Laboratory Test Bench
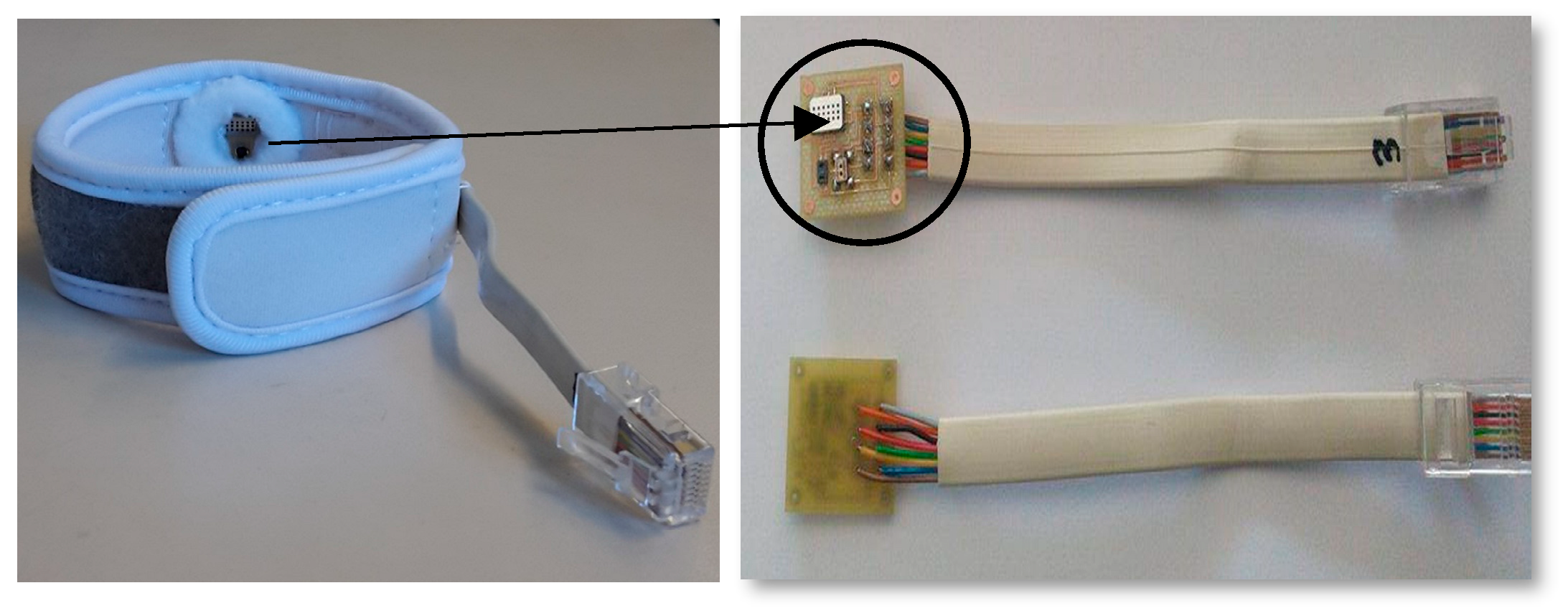
4. Clinical Trials
4.1. Aim
4.2. Clinical Protocol
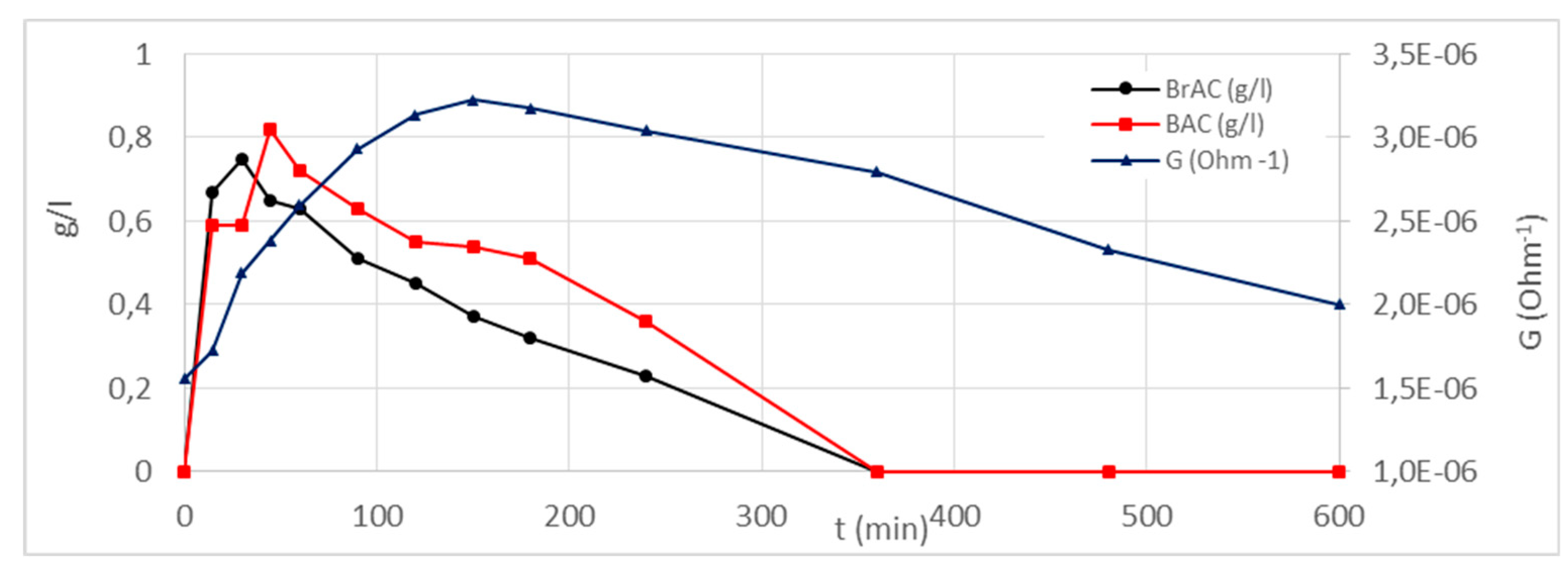
4.3. Results and Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mochalski, P.; King, J. Emission rates of selected volatile organic compounds from skin of healthy volunteers. J. Chromatogr. B 2014, 959, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.M.; McKnight, A.S. Evaluation Transdermal Alcohol Measuring Device; Pacific Institute for research and Evaluation: Calverton, NY, USA, 2007. [Google Scholar]
- Widmark, E.M.P. Principles and Applications of Medico Legal Alcohol Determination; English translation of 1932 German ed.; Davis Biomedical Publications:: Davis, CA, USA, 1981. [Google Scholar]
- Batt, R.D. Adsorption, distribution and elimination of alcohol. In Human Metabolism of Alcohol; Crow, K.E., Batt, R.D., Eds.; CRC Press: Boca Raton, FL, USA, 1989; Volume I. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawson, B.; Martini-Laithier, V.; Fiorido, T.; Annanouch, F.; Burtey, S.; Cassé-Perrot, C.; Audebert, C.; Bendahan, M.; Bouchakour, R.; Blin, O.; et al. Transdermal Alcohol Measurements Using MOX Sensors in Clinical Trials. Proceedings 2017, 1, 431. https://doi.org/10.3390/proceedings1040431
Lawson B, Martini-Laithier V, Fiorido T, Annanouch F, Burtey S, Cassé-Perrot C, Audebert C, Bendahan M, Bouchakour R, Blin O, et al. Transdermal Alcohol Measurements Using MOX Sensors in Clinical Trials. Proceedings. 2017; 1(4):431. https://doi.org/10.3390/proceedings1040431
Chicago/Turabian StyleLawson, Bruno, Virginie Martini-Laithier, Tomas Fiorido, Fatima Annanouch, Stephane Burtey, Catherine Cassé-Perrot, Christine Audebert, Marc Bendahan, Rachid Bouchakour, Olivier Blin, and et al. 2017. "Transdermal Alcohol Measurements Using MOX Sensors in Clinical Trials" Proceedings 1, no. 4: 431. https://doi.org/10.3390/proceedings1040431
APA StyleLawson, B., Martini-Laithier, V., Fiorido, T., Annanouch, F., Burtey, S., Cassé-Perrot, C., Audebert, C., Bendahan, M., Bouchakour, R., Blin, O., & Aguir, K. (2017). Transdermal Alcohol Measurements Using MOX Sensors in Clinical Trials. Proceedings, 1(4), 431. https://doi.org/10.3390/proceedings1040431




