ZnO/SnO2 Heterojunctions Sensors with UV-Enhanced Gas-Sensing Properties at Room Temperature †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Characterization Techniques
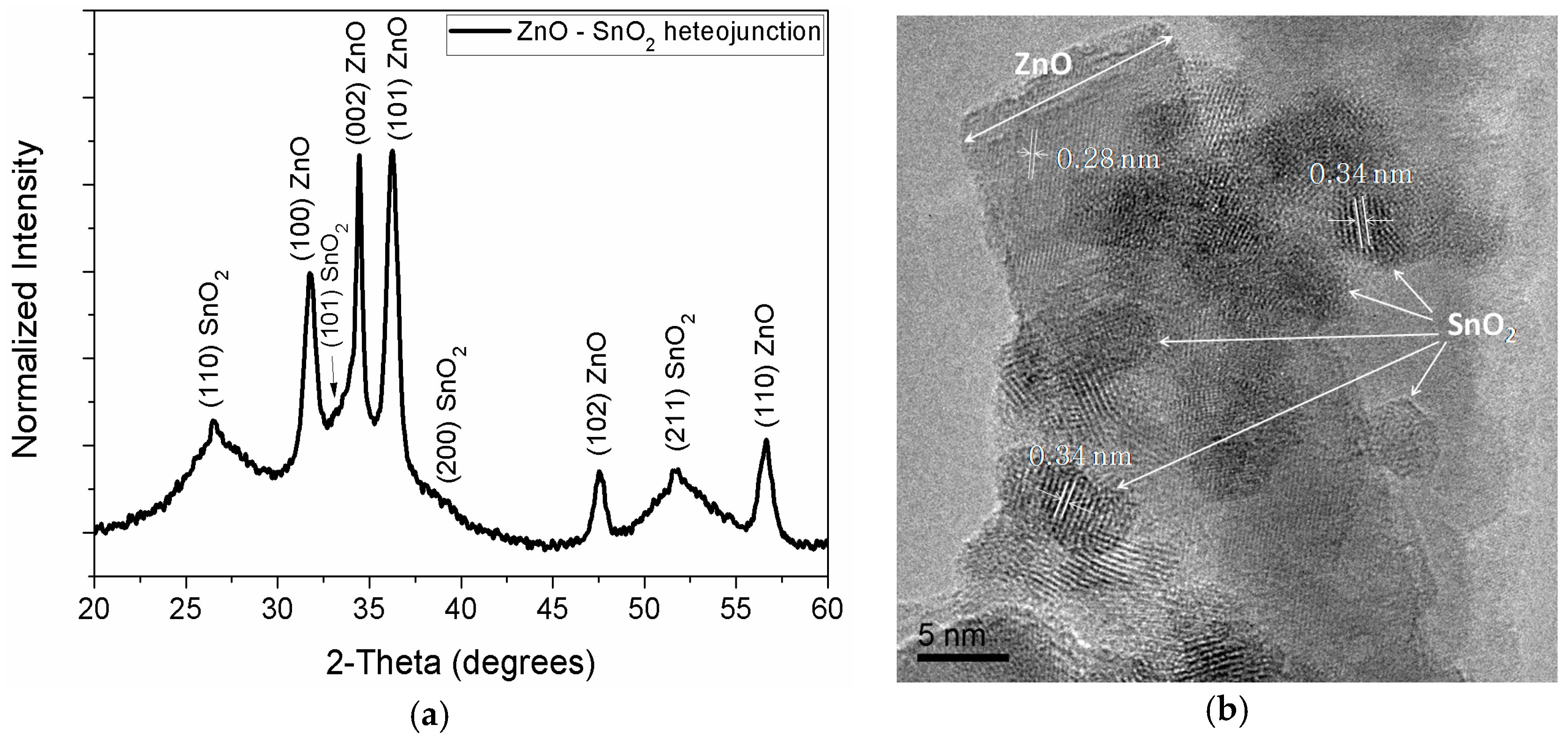
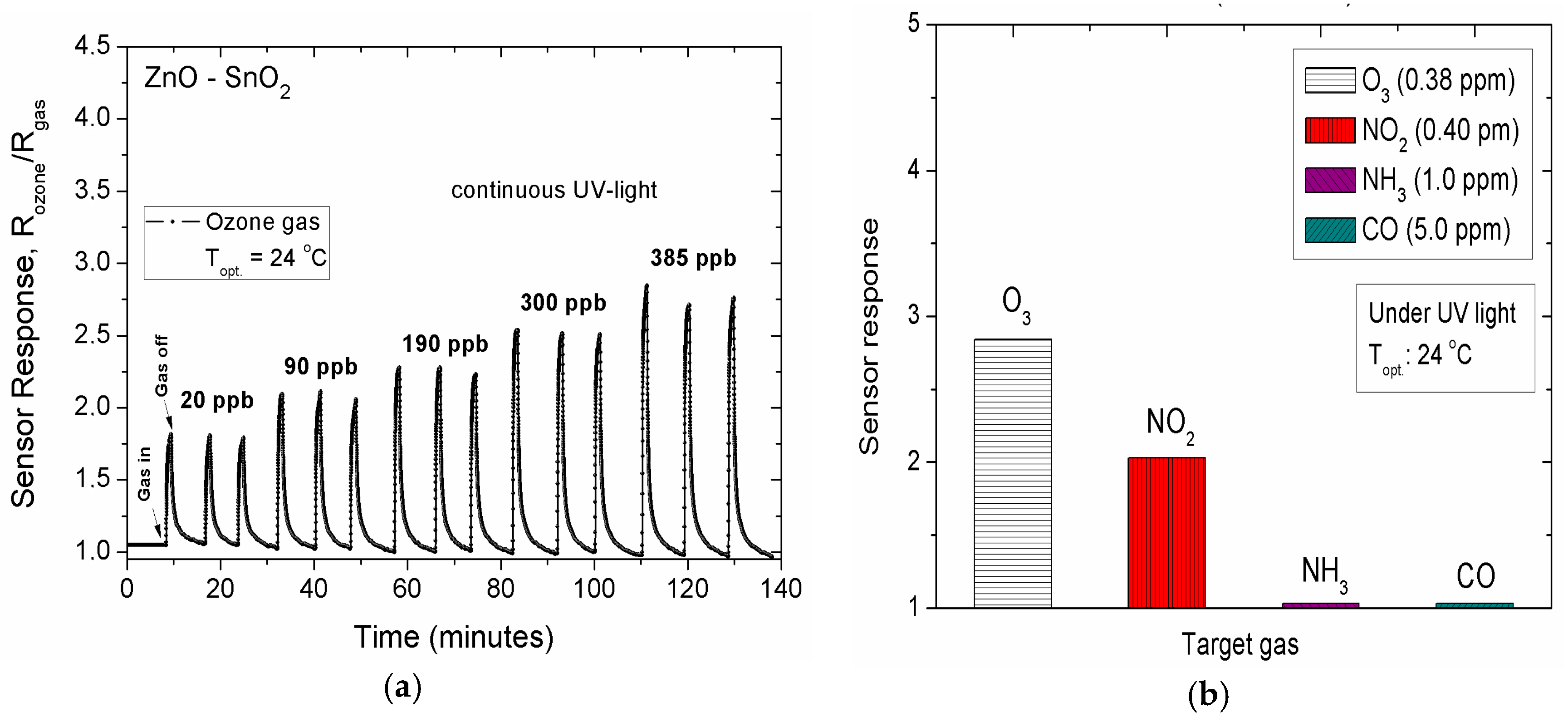
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent progress on the development of chemosensors for gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef] [PubMed]
- Janáky, C.; Rajeshwar, K.; de Tacconi, N.R.; Chanmanee, W.; Huda, M.N. Tungsten-based oxide semiconductors for solar hydrogen generation. Catal. Today 2013, 199, 53–64. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Toupance, T.; Servant, L.; Müller, M.M.; Kleebe, H.-J.; Ziegler, J.; Jaegermann, W. Nanostructured SnO2–ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes. Inorg. Chem. 2012, 51, 7764–7773. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shang, L.; Chen, S.; Xia, J.; Qi, X.; Wang, X.; Zhang, T.; Meng, X.-M. Type-II ZnO nanorod-SnO2 nanoparticle heterostructures: characterization of structural, optical and photocatalytic properties. Nanoscale 2013, 5, 3828–3833. [Google Scholar] [CrossRef] [PubMed]
- Catto, A.C.; da Silva, L.F.; Bernardi, M.I.B.; Bernardini, S.; Aguir, K.; Longo, E.; Mastelaro, V.R. Local structure and surface properties of CoxZn1–xO thin films for ozone gas sensing. ACS Appl. Mater. Interfaces 2016, 8, 26066–26072. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Faglia, G.; Sberveglieri, G. UV light activation of tin oxide thin films for NO2 sensing at low temperatures. Sens. Actuators B Chem. 2001, 78, 73–77. [Google Scholar] [CrossRef]
- Fan, S.-W.; Srivastav, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Da Silva, L.F.; M’Peko, J.-C.; Catto, A.C.; Bernardini, S.; Mastelaro, V.R.; Aguir, K. Ribeiro, C.; Longo, E. UV-enhanced ozone gas sensing response of ZnO-SnO2 heterojunctions at room temperature. Sens. Actuators B Chem. 2017, 240, 573–579. [Google Scholar] [CrossRef]
- Park, S.; An, S.; Mun, Y.; Lee, C. UV-enhanced NO2 gas sensing properties of SnO2-Core/ZnO-shell nanowires at room temperature. ACS Appl. Mater. Interfaces 2013, 5, 4285–4292. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.F.; Lopes, O.F.; Catto, A.C.; Avansi, W., Jr.; Bernardi, M.I.B.; Li, M.S.; Ribeiro, C.; Longo, E. Hierarchical growth of ZnO nanorods over SnO2 seed layer: Insights into electronic properties from photocatalytic activity. RSC Adv. 2016, 6, 2112–2118. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.F.d.; Lucchini, M.A.; M’Peko, J.-C.; Bernardini, S.; Aguir, K.; Ribeiro, C.; Longo, E.; Niederberger, M. ZnO/SnO2 Heterojunctions Sensors with UV-Enhanced Gas-Sensing Properties at Room Temperature. Proceedings 2017, 1, 418. https://doi.org/10.3390/proceedings1040418
Silva LFd, Lucchini MA, M’Peko J-C, Bernardini S, Aguir K, Ribeiro C, Longo E, Niederberger M. ZnO/SnO2 Heterojunctions Sensors with UV-Enhanced Gas-Sensing Properties at Room Temperature. Proceedings. 2017; 1(4):418. https://doi.org/10.3390/proceedings1040418
Chicago/Turabian StyleSilva, Luís F. da, Mattia A. Lucchini, Jean-Claude M’Peko, Sandrine Bernardini, Khalifa Aguir, Caue Ribeiro, Elson Longo, and Markus Niederberger. 2017. "ZnO/SnO2 Heterojunctions Sensors with UV-Enhanced Gas-Sensing Properties at Room Temperature" Proceedings 1, no. 4: 418. https://doi.org/10.3390/proceedings1040418
APA StyleSilva, L. F. d., Lucchini, M. A., M’Peko, J.-C., Bernardini, S., Aguir, K., Ribeiro, C., Longo, E., & Niederberger, M. (2017). ZnO/SnO2 Heterojunctions Sensors with UV-Enhanced Gas-Sensing Properties at Room Temperature. Proceedings, 1(4), 418. https://doi.org/10.3390/proceedings1040418








