A Non Invasive Sensor System for the Screening of Obstructive Sleep Apnea Syndrome †
Abstract
:1. Introduction
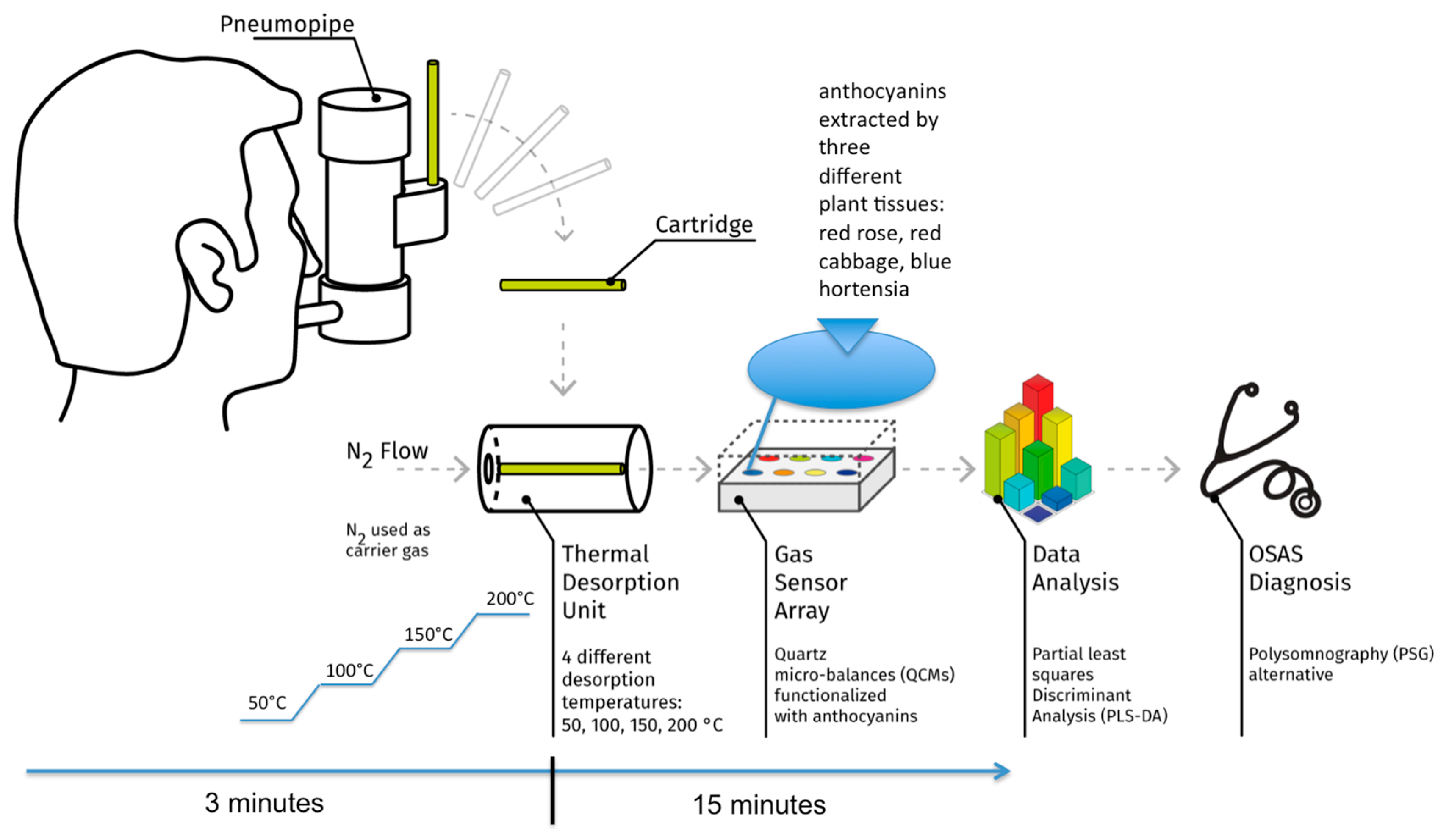
2. Materials and Methods
3. Results
4. Discussion
Acknowledgments
Conflicts of Interest
References
- Sánchez, M.; Campos-Rodriguez, F.; Barbé, F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir. Med. 2013, 1, 61–72. [Google Scholar] [CrossRef]
- Greulich, T.; Hattesohl, A.; Grabisch, A.; Koepke, J.; Schmid, S.; Noeske, S.; Nell, C.; Wencker, M.; Jörres, R.A.; Vogelmeier, C.F.; et al. Detection of obstructive sleep apnoea by an electronic nose. Eur. Respir. J. 2013, 42, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Antonelli Incalzi, R.; Pennazza, G.; Scarlata, S.; Santonico, M.; Vernile, C.; Cortese, L.; Frezzotti, E.; Pedone, C.; D’Amico, A. Comorbidity modulates non invasive ventilation-induced changes in breath print of obstructive sleep apnea syndrome patients. Sleep Breath. 2015, 19, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Pennazza, G.; Santonico, M.; Incalzi, R.A.; Scarlata, S.; Chiurco, D.; Vernile, C.; D’Amico, A. Measure chain for exhaled breath collection and analysis: A novel approach suitable for frail respiratory patients. Sens. Actuators B Chem. 2014, 204, 578–587. [Google Scholar] [CrossRef]
- Santonico, M.; Pennazza, G.; Grasso, S.; D’Amico, A.; Bizzarri, M. Design and test of a biosensor-based multisensorial system: A proof of concept study. Sensors 2013, 13, 16625–16640. [Google Scholar] [CrossRef] [PubMed]
| Hypoxic OSAS | Non Hypoxic OSAS | COPD | |
|---|---|---|---|
| Hypoxic OSAS | 6 | 4 | 1 |
| Non hypoxic OSAS | 0 | 13 | 4 |
| COPD | 3 | 6 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennazza, G.; Santonico, M.; Scarlata, S.; Santangelo, S.; Grasso, S.; Zompanti, A.; Incalzi, R.A. A Non Invasive Sensor System for the Screening of Obstructive Sleep Apnea Syndrome. Proceedings 2017, 1, 426. https://doi.org/10.3390/proceedings1040426
Pennazza G, Santonico M, Scarlata S, Santangelo S, Grasso S, Zompanti A, Incalzi RA. A Non Invasive Sensor System for the Screening of Obstructive Sleep Apnea Syndrome. Proceedings. 2017; 1(4):426. https://doi.org/10.3390/proceedings1040426
Chicago/Turabian StylePennazza, Giorgio, Marco Santonico, Simone Scarlata, Simona Santangelo, Simone Grasso, Alessandro Zompanti, and Raffaele Antonelli Incalzi. 2017. "A Non Invasive Sensor System for the Screening of Obstructive Sleep Apnea Syndrome" Proceedings 1, no. 4: 426. https://doi.org/10.3390/proceedings1040426






