1. Introduction
Precision agriculture can help to reduce the environmental impacts of intensive agriculture by determining fertilizers doses over time and at small scale according to soils variability. Thus, our project aims to provide a low-cost, robust and real-time multimodal tool based on ISFET microsensors for in-situ monitoring of soil nutrients.
Since the 2000s, several studies showed the promising future of potentiometric sensors in precision agriculture. Most of them focus on the development of flow injection analysis (FIA) systems in which ion-selective electrodes (ISE) or ISFET are used for nutrients measurements in soil extracts performed on-site or on-the-go [
1,
2]. Artigas et al. reported the fabrication of pH-ISFET, pK-ISFET, pCa-ISFET and pNO
3-ISFET used directly in soil [
3]. Nevertheless, more studies are needed to fully assess some important parameters of ISFET’s detection capability in soil: influence of soil moisture, ISFET drift and lifetimes and the in-situ detection of other important nutrients like NH
4+ ammonium ions.
2. Microsensor Fabrication and Measurement Methods
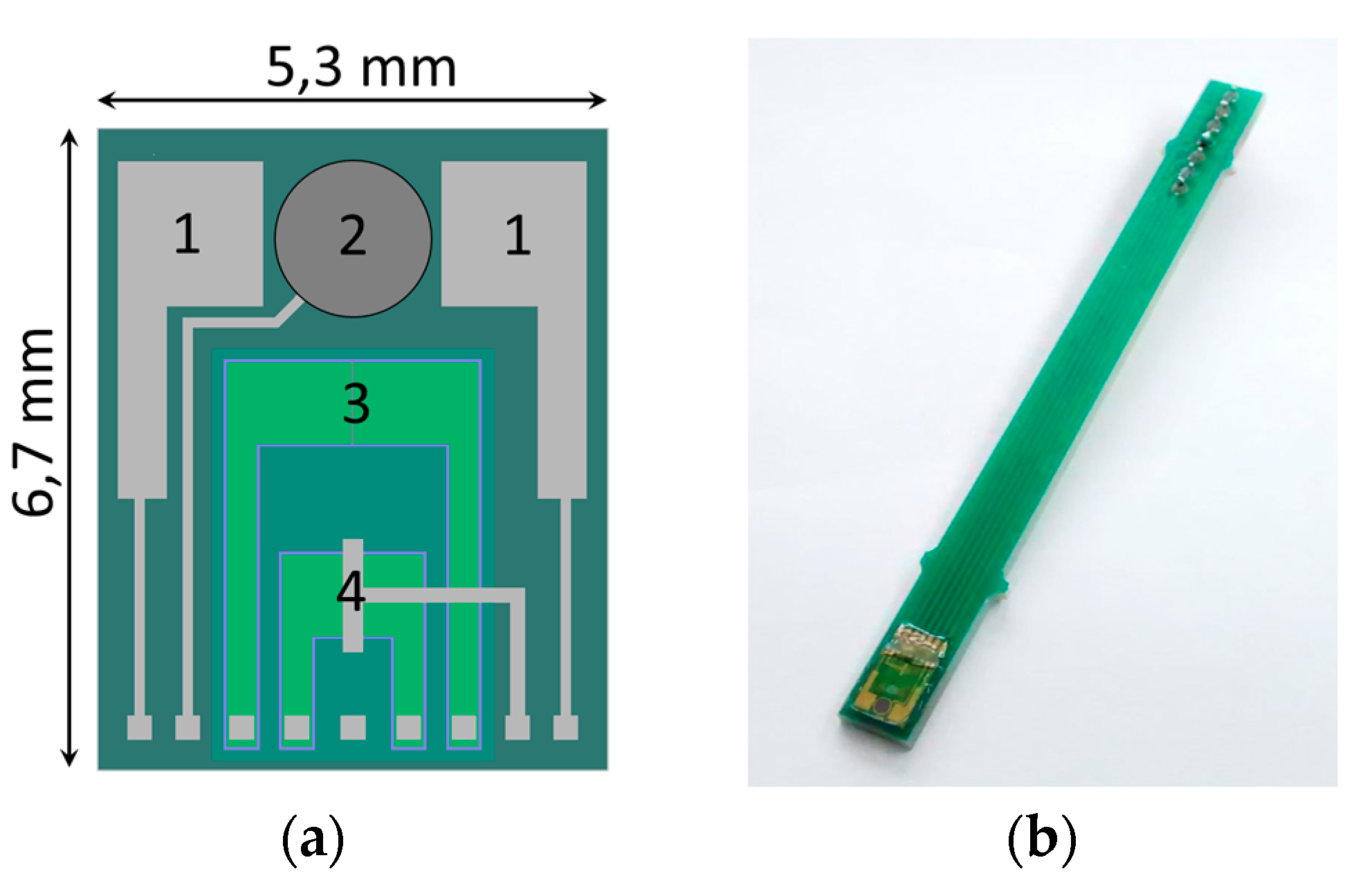
Our microsystem is fabricated on 4-inch silicon substrates and composed of an ISFET, a miniature solid-state reference electrode, two platinum electrodes for EC measurements and a temperature sensor in MOSFET technology (
Figure 1a).
N-channel, normally-off pH-ISFET microsensors were fabricated using a 50 nm silicon nitride Si
3N
4 pH-sensitive layer deposited by Low Pressure Chemical Vapor Deposition (LPCVD) on top of a 50 nm thermal oxide SiO
2 layer. Such ISFET was already studied in previous work, evidencing a quasi-Nernstian sensitivity of around 50 mV/pH [
4].
The detections of ammonium and nitrate ions were allowed by casting 0.5 µL of an ion-sensitive membrane on top of the pH-ISFET Si3N4 layer. The membranes were based on fluoropolysiloxane (FPSX) matrix sonicated in tetrahydrofuran with ionophores and ionic additives. It contained: 3.5% (w/w) nonactin, 1.5% (w/w) KTpClPB, 95.0% (w/w) FPSX in the case of pNH4-ISFET; 1.5% (w/w) Nitrate ionophore V, 0.9% (w/w) TDMACl, 97.6% (w/w) FPSX in the case of pNO3-ISFET. The FPSX was chosen for its good adhesion properties on Si3N4 surface leading to an improved sensor’s lifetime.
The performances of the ISFETs were examined using a specific potentiometric ISFET-meter electronic interface with drain voltage Vds and current Id fixed to 2.0 V and 0.1 mA respectively. A silver silver chloride double junction electrode was used as a reference electrode in aqueous solution and in soils. Sensitivity tests were performed by increasing the activity of the primary ions using a 0.1 M ammonium nitrate NH4NO3 aqueous solution. Selectivity coefficients were obtained with the Fixed Interference Method (FIM). Measurements in soils were done by inserting the ISFET’s PCB directly into the soil in a way that the sensitive part of the sensor was in close contact with the soil. All measurements were carried out at room temperature.
3. Results and Discussion
3.1. In-Situ Detection of Soil pH
We first studied the detection capability of pH-ISFET microsensors inserted directly into the soil in order to prove the feasibility of a direct measurement method as opposed to soil extract analysis.
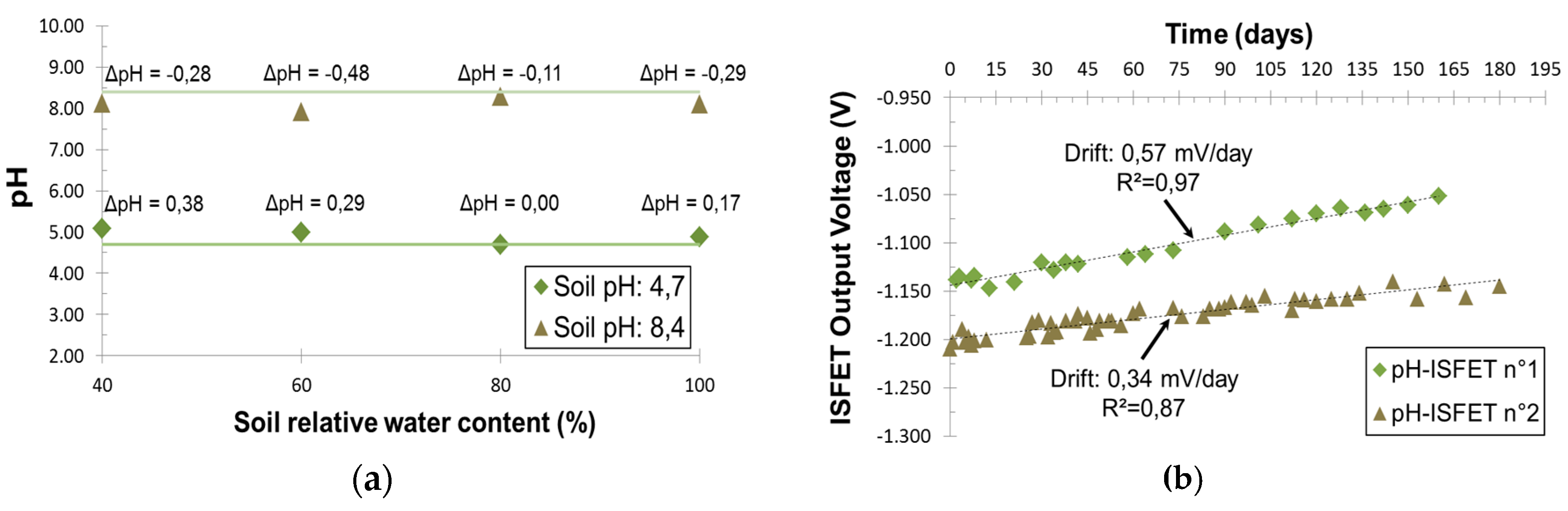
Two silty-clay soils were first analyzed according to the standard method where a glass pH electrode is used in a 20 vol % suspension of soil in water. It led to pH values of 4.7 and 8.4. After a three points-calibration in pH buffer solutions (pH 4, 7 and 10), quick in-situ measurements of pH-ISFET were recorded for both soils at different soil moisture levels (
Figure 2a). The results are in great accordance with the standard method. Indeed, the maximum error was only 0.5 pH unit and there was no correlation with soil moisture. In-situ measurements were even possible at soil relative water content of 40% which is below typical wheat growths conditions. With its small measurement errors relative to an agriculture application and its robustness, pH-ISFET is fully compatible for quick on-field pH determination.
Furthermore, we recorded for six months the signal drift of two pH-ISFET directly inserted in pots of silty-clay soil using the simple packaging showed on
Figure 2b. This study aimed at assessing the pH-ISFET lifetime in soil to know if a monitoring during all wheat growth stages could be possible. Relative water content (75%) was kept constant thanks to a daily subirrigation. Both microsensors displayed a linear (R
2 ≥ 0.87) and relatively small drift (<0.6 mV/day). The ageing of pH-ISFET caused by soil environment was evaluated by a comparison of their sensitivity before and after the experiment. Both ISFET kept their initial sensitivity (51 mV/pH). Thus, our pH-ISFET showed a lifetime in soil of at least six months making the project of a long-term monitoring of soil nutrients possible.
3.2. In-Vitro Detection of Nitrate and Ammonium Ions
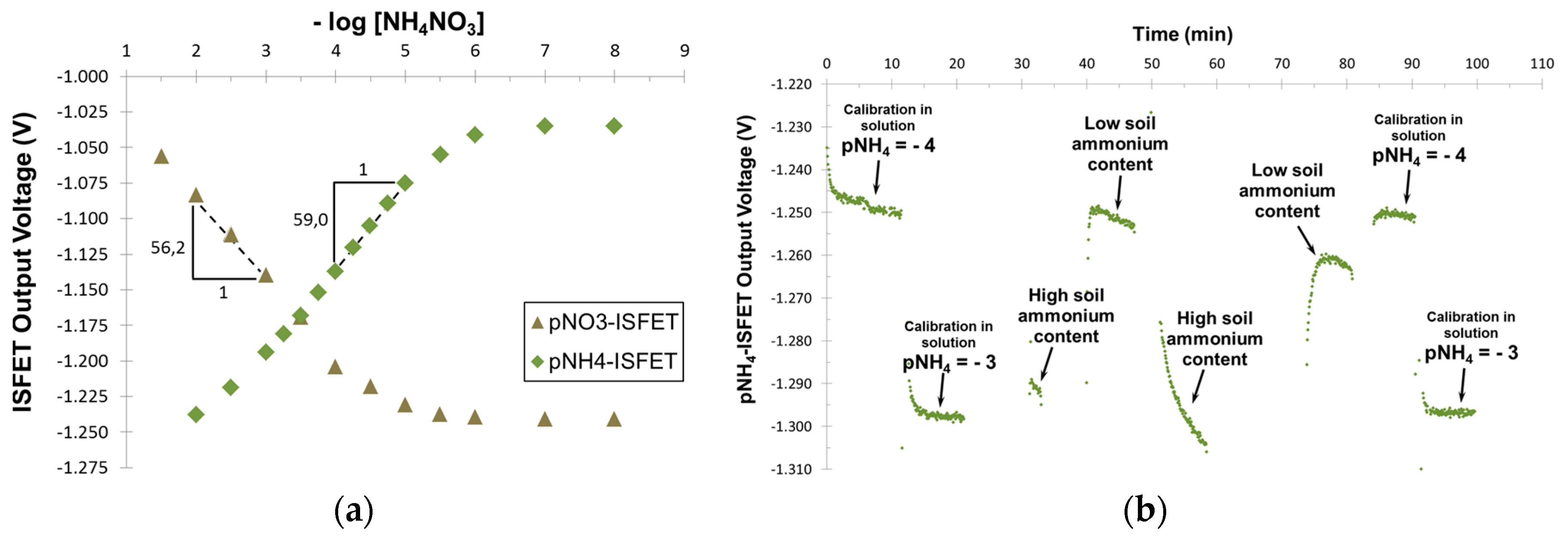
Calibration curves obtained for pNO
3- and pNH
4-ISFET are shown in
Figure 3a. Both sensors exhibited a linear response to the concentration of primary ions with a quasi-Nernstian sensitivity of 59.0 mV/pNH
4 and 56.2 mV/pNO
3, respectively. The limits of detection (LOD) calculated from the intersection of the two extrapolated segments of the calibration curve, were 3.2 µM and 17 µM, respectively. Both the LOD and the linear range are in accordance with standard values of mineral nitrogen in field conditions. Thus, our probe should meet the requirements for a soil study application.
A specific care was taken for the determination of FIM selectivity coefficient (
Table 1) as direct measurements in soils could obviously not be done without the presence of interfering ions.
Overall, both functionalized ISFET exhibited good selectivity coefficients towards the main ions likely to be found in soil. As expected with nonactin ionophore, potassium K+ ion interfered the strongest with our pNH4-ISFET. Despite the use of Nitrate Ionophore V, the relative responses of our pNO3-ISFET towards interfering ions indicate a Hofmeister behavior with a poor selectivity towards chloride. Nevertheless, with exception to coastal soils in which high content in chloride is expected, our pNO3-ISFET should be selective enough for an application to soil monitoring.
3.3. In-Situ Detection of Ammonium Ions
Finally, we tested our pNH
4-ISFET in silty-clay soils of varying nitrogen content. Starting with two pots of the same soil, we obtained two distinct nitrogen content soils by putting them respectively at 38 °C and 4 °C for one month. Indeed, nitrogen mineralization by soil microorganisms is a temperature-dependent process. Measurements performed into these soils directly after calibration allowed to discriminate the two levels of nitrogen. We successfully recorded an increase in ammonium concentration caused by the stimulation of nitrogen mineralization by soil microorganisms (
Figure 3b).
4. Conclusions
Our pH-ISFET succeeded in measuring acidic and basic soils, for short and long periods of time, with only an elementary packaging and in avoiding the need for a time-consuming step of soil sampling. These results validated the feasibility of a direct measurement method in soil. Then, our nitrogen-functionalized ISFET exhibited quasi-Nernstian sensitivity and detection limit in accordance with standard values in field conditions. Our pNH4-ISFET was able to differentiate two soils of different nitrogen content. Overall, this work highlights the promising future of the ISFET technology for precision agriculture.