NAP-XPS Study of Ethanol Adsorption on TiO2 Surfaces and Its Impact on Microwave-Based Gas Sensors Response †
Abstract
:1. Introduction
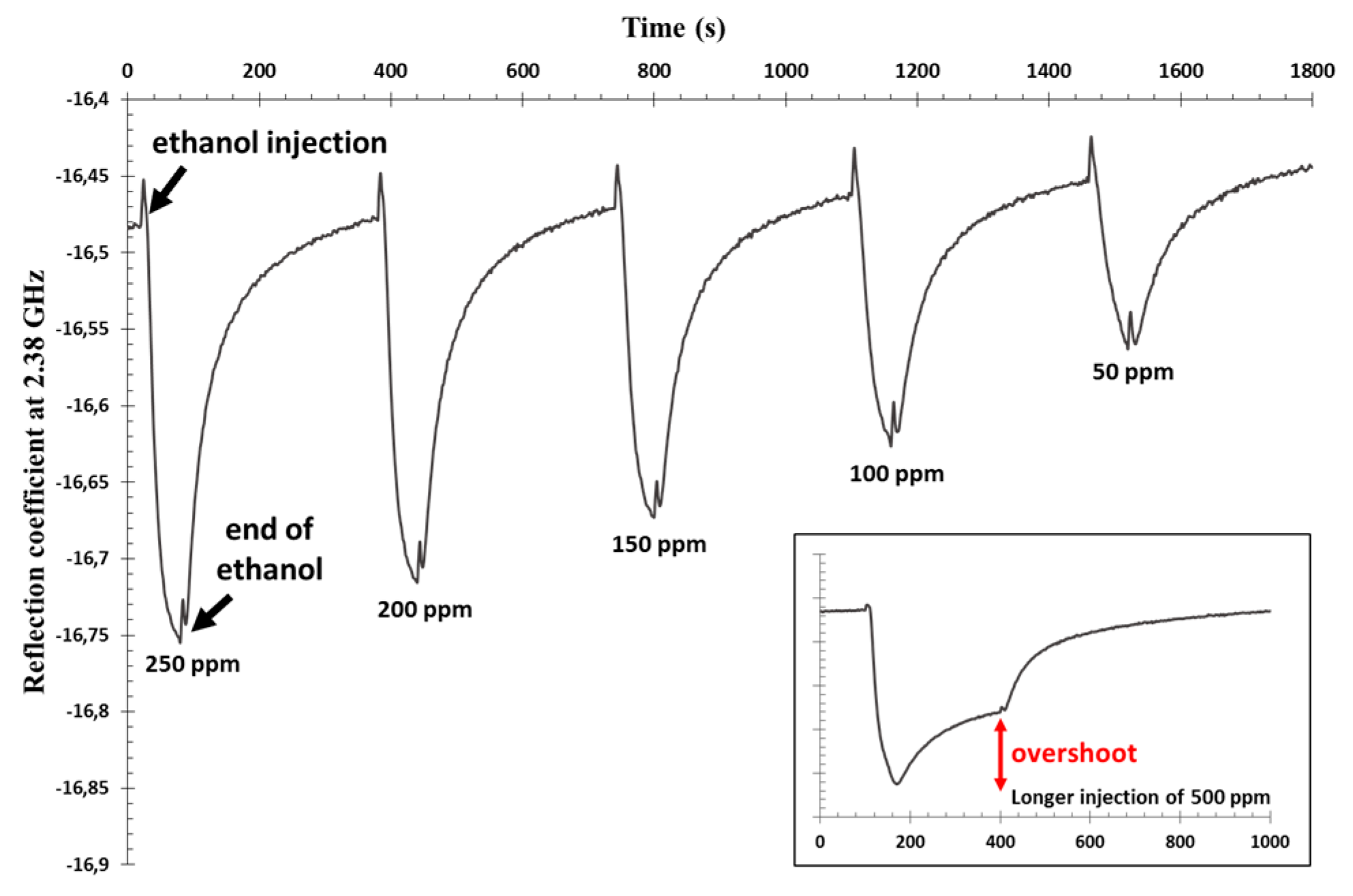
2. Microwave Experiment
3. NAP-XPS Study of Ethanol Adsorption
Acknowledgments
Conflicts of Interest
References
- De Fonseca, B.; Rossignol, J.; Stuerga, D.; Pribetich, P. Microwave signature for gas sensing: 2005 to present. Urban Clim. 2015, 14, 502–515. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable sensors for ubiquitous monitoring of gases in the era of internet of things and industrial internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef] [PubMed]
- Bailly, G.; Harrabi, A.; Rossignol, J.; Michel, M.; Stuerga, D.; Pribetich, P. Microstrip spiral resonator for microwave-based gas sensing. IEEE Sens. Lett. 2017. [Google Scholar] [CrossRef]
- Bailly, G.; Harrabi, A.; Rossignol, J.; Stuerga, D.; Pribetich, P. Microwave gas sensing with a microstrip interdigital capacitor: Detection of NH3 with TiO2 nanoparticles. Sens. Actuators B 2016, 236, 554–564. [Google Scholar] [CrossRef]
- Bakrania, S.D.; Wooldridge, M.S. The effects of two thick film deposition methods on tin dioxide gas sensor performance. Sensors 2009, 9, 6853–6868. [Google Scholar] [CrossRef] [PubMed]
- Murcia, J.J.; Hidalgo, M.C.; Navío, J.A.; Araña, J.; Doña-Rodríguez, J.M. In situ FT-IR study of the adsorption and photocatalytic oxidation of ethanol over sulfated and metallized TiO2. Appl. Catal. B Environ. 2016, 142–143, 205–213. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, G.; Rossignol, J.; Stuerga, D.; Pribetich, P.; Domenichini, B. NAP-XPS Study of Ethanol Adsorption on TiO2 Surfaces and Its Impact on Microwave-Based Gas Sensors Response. Proceedings 2017, 1, 416. https://doi.org/10.3390/proceedings1040416
Bailly G, Rossignol J, Stuerga D, Pribetich P, Domenichini B. NAP-XPS Study of Ethanol Adsorption on TiO2 Surfaces and Its Impact on Microwave-Based Gas Sensors Response. Proceedings. 2017; 1(4):416. https://doi.org/10.3390/proceedings1040416
Chicago/Turabian StyleBailly, Guillaume, Jerome Rossignol, Didier Stuerga, Pierre Pribetich, and Bruno Domenichini. 2017. "NAP-XPS Study of Ethanol Adsorption on TiO2 Surfaces and Its Impact on Microwave-Based Gas Sensors Response" Proceedings 1, no. 4: 416. https://doi.org/10.3390/proceedings1040416
APA StyleBailly, G., Rossignol, J., Stuerga, D., Pribetich, P., & Domenichini, B. (2017). NAP-XPS Study of Ethanol Adsorption on TiO2 Surfaces and Its Impact on Microwave-Based Gas Sensors Response. Proceedings, 1(4), 416. https://doi.org/10.3390/proceedings1040416




