1. Introduction
Nitrogen oxides (NO
2), emitted from the coil combustion, industrial production and vehicle emissions, give rise to acid rain and photochemical smog. It is highly toxic to the health of human and harmful to the environment, so the detection of NO
2 is urgently desired. As a consequence, the development of the gas sensor used for NO
2 detection attracted widespread attention. Among them, metal oxide semiconductors (MOS) are widely studied on account of its high sensitivity, low cost and low detection limit [
1,
2,
3]. However, traditional metal oxides work at relatively high temperature, in the range of 150–450°, which causes many problems, such as increasing the fabrication cost, deteriorating the sensing performance. Recently, room temperature gas sensors have become very attractive since they can be operated without heating, and thus simplifying the sensor design, reducing the fabrication cost, decreasing the power consumption and increasing the long-term stability. The main issue for the development of room-temperature gas sensors is to prepare sensitive materials with specific characteristics. The current trend is the development of materials with high specific surface area, low-dimensional nanostructure, rich oxygen deficiency or p-n heterojunctions. Apart from semiconductors, graphene is a well-known two-dimensional material with large specific surface area, high conductivity and good optical absorption properties [
1,
4]. Constructing a metal oxide and graphene composite may be a feasible approach to greatly enhance the gas sensing performance. In this study, we propose a facile one-step hydrothermal method to prepare a binary composite of reduced graphene oxide (rGO)/oxygen-deficient (ZnO
1−x) hybrids.
3. Results and Discussion
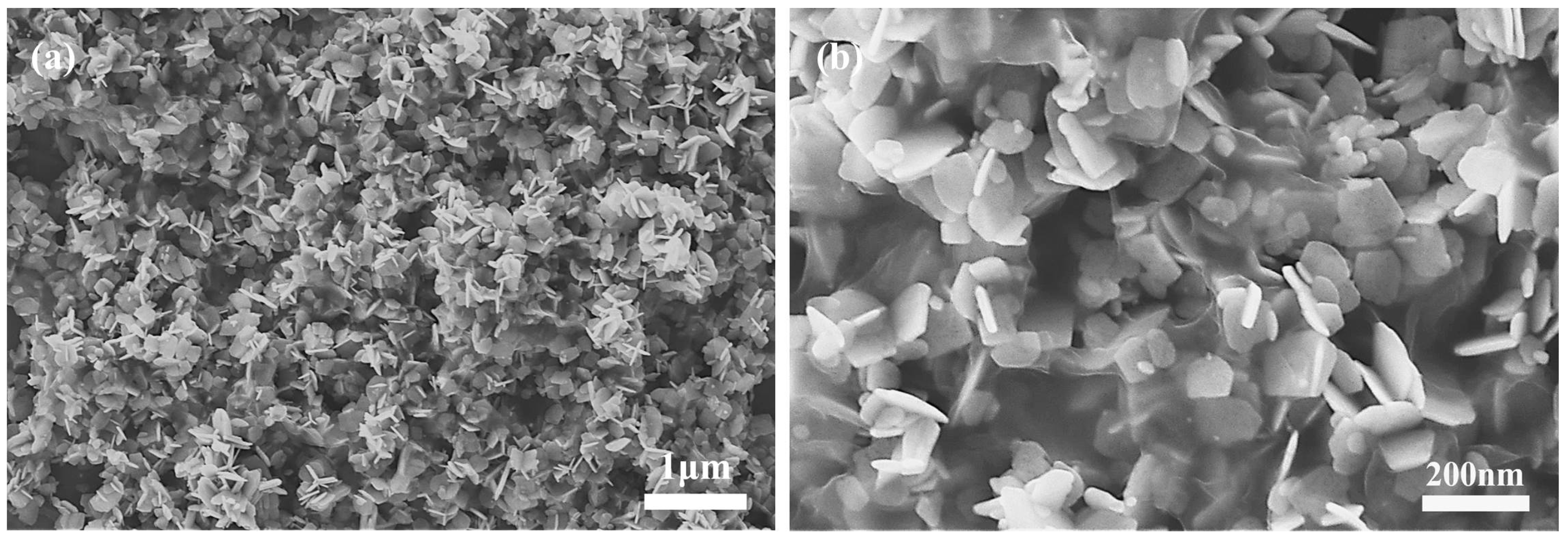
From the FE-SEM images of the as-prepared rGO@ZnO
1−x composites (
Figure 1), the composites show a sheet-like morphology with a size of ten to several hundred nanometers. The synthesized rGO exhibits a gauze-like sheet morphology. The ZnO sheets are enwrapped by gauze-like rGO.
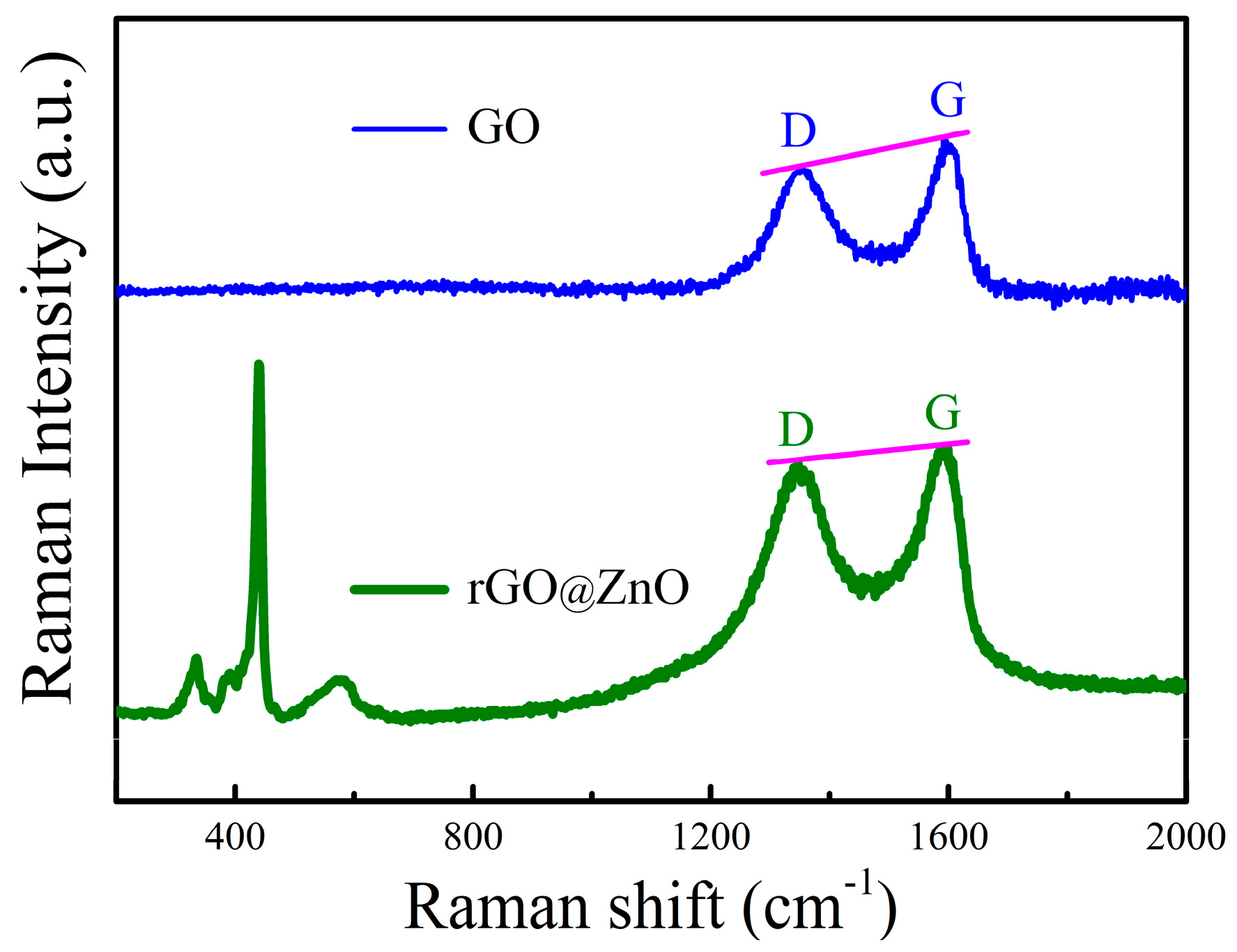
In addition to the morphology, the phase composition and structure of the samples are also needed to identify. For the carbon-based materials, such as graphene, is very suitable to adopt Raman technique to characterize.
The Raman spectra of the as-prepared composites ranging from 200 to 2000 cm
−1 are illustrated in
Figure 2. Pure GO suspension exhibits two signals located at 1354 and 1590 cm
−1, respectively. According to the previous study [
1,
4], the signal centered at 1354 cm
−1 arises from the D band, and the 1590 cm
−1 one is ascribed to the G band. For the as-synthesized composite materials, the D (1351 cm
−1) and G band (1589 cm
−1) also exists. Further studies show that the I
D/I
G (intensity ratio of D and G band) of the composites (0.97) is higher than that of GO (0.88). It means that GO is reduced to rGO, similar results are also reported in [
4]. In addition to the D and G signals, other peaks suited at 330, 384, 437 and 588 cm
−1, which are the characteristic peaks of ZnO, are also present. The 588 cm
−1 peak is associated with the oxygen vacancies, meaning that the ZnO
1−x phase is formed.
To overcome the problem that metal oxides based gas sensors are needed to operate at high temperature, we adopt light illumination to activate the sensing materials instead of heating. Pure stoichiometric ZnO is well known to be a kind of wide bandgap (3.37 eV) semiconductor, so it can only absorb UV lights. Only UV lights can excite ZnO and generate many free electron-hole pairs at room temperature. The detection of target gases for semiconductor sensors is based on the change in electron concentration, which means that pure ZnO cannot work with other lights except UV lights. In practical white lights, just a small part is UV lights and most of them are visible lights. In order to make full use of the white lights, it is required to extend the light absorption range by constructing ZnO based composites. From the previous studies [
1,
2,
3], we found that the optical absorption range of the sensitive materials is highly related to the final gas sensing properties. As a result, it is imperative to investigate the optical properties of the materials.
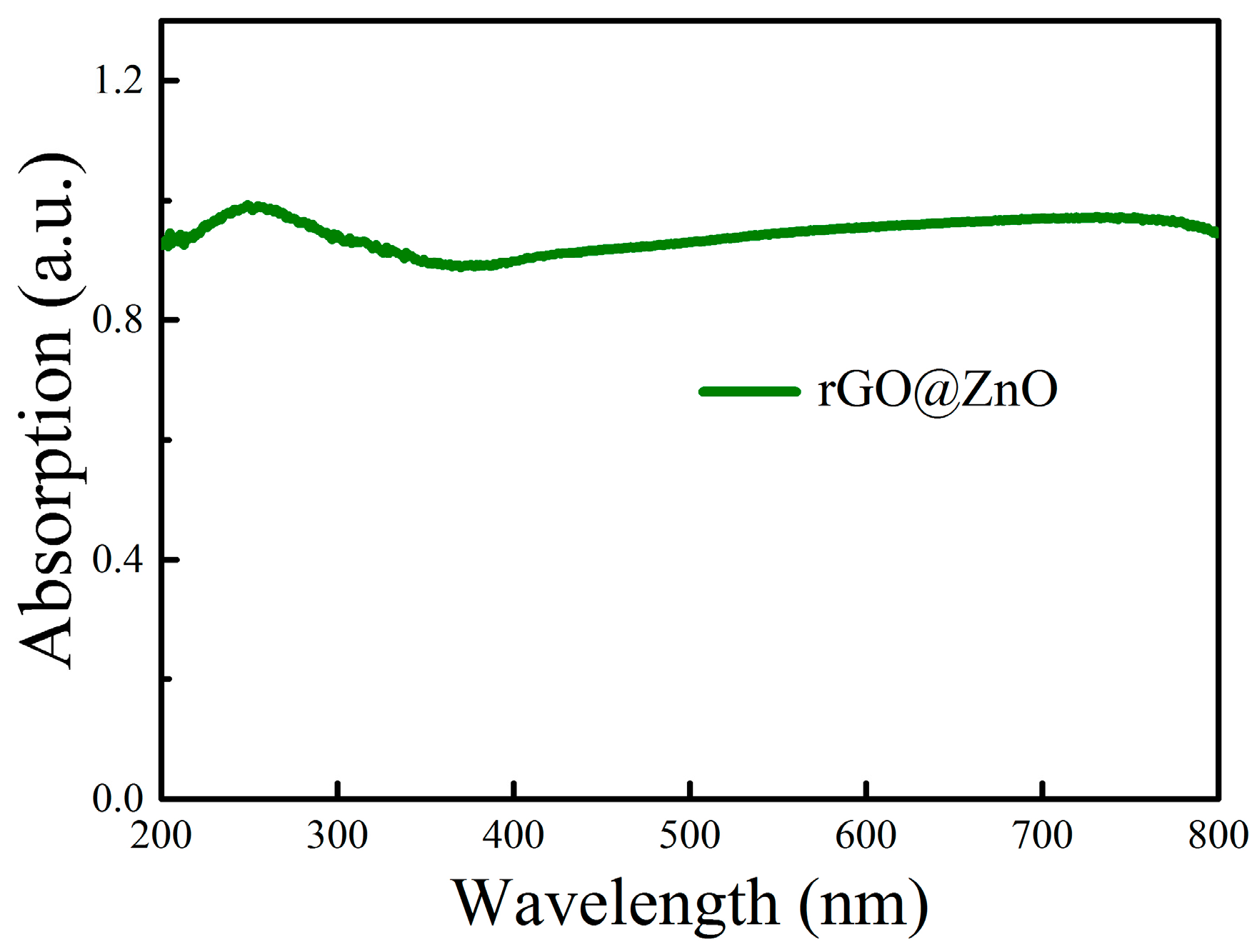
Figure 3 illustrates the UV-Vis absorption spectra of the as-synthesized composites, and it can be found that it has strong absorption in the whole visible and UV light area. It indicates that there are highly concentrated free electrons in the conduction band of the rGO@ZnO
1−x composites upon exposure to white light irradiation.
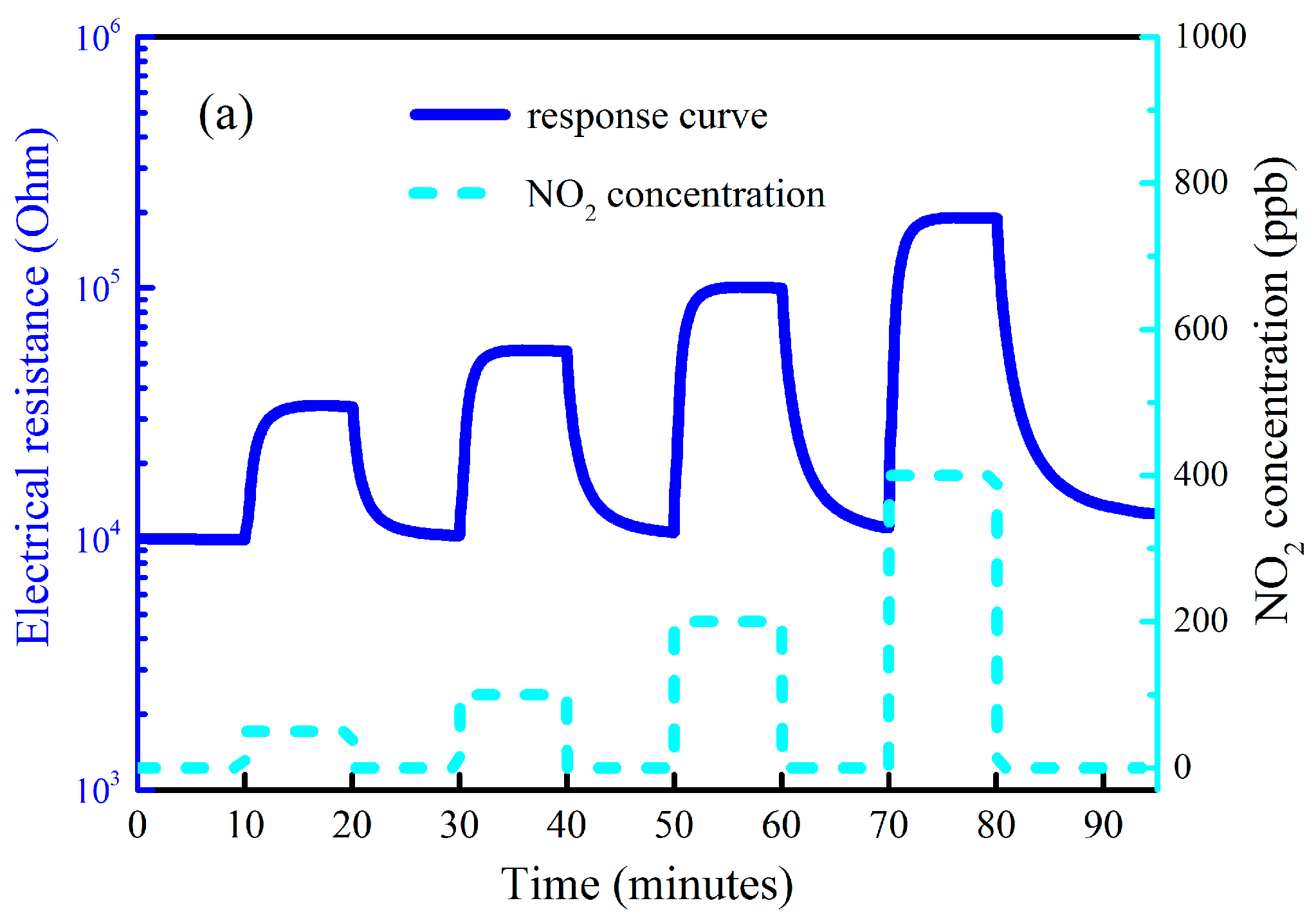
Figure 4 depicts the gas sensing curve for the as-synthesized rGO@ZnO
1−x composites towards NO
2 in the concentration range of 50–400 ppb with white light stimulation at room temperature. Upon exposure to NO
2 gas, the electrical resistance of the sensitive materials increases, which is a typical behavior of n-type semiconductors. From
Figure 4, the sensor response is 2.31, 4.66, 9.15 and 18.67 when the NO
2 concentration is 50, 100, 200 and 400 ppb, respectively. It is interesting to find that the sensor response is as high as 2.31 even for 50 ppb NO
2, so the detection limit of this presented sensors is very low. The enhanced gas sensing properties are attributed to the large specific surface area, high oxygen defects concentration, p-n heterojunctions, high carrier mobility and extended visible light absorption range of the as-prepared materials. Graphene is well-known to be a two-dimensional nanomaterial with the large specific surface area, so the surface area of the rGO@ZnO
1−x composites is much higher compared with that of pure ZnO. In addition, oxygen vacancies created on ZnO surface are preferential adsorption sites for oxidizing gases [
5,
6]. When the light is on, the composites are excited, and many electron-hole pairs are produced. Due to the presence of ZnO and rGO, the electrons will transfer from rGO to ZnO, while holes will move in the opposite direction, which significantly prevents the recombination of electron-hole pairs and prolong the life of free electrons. More free electrons can participate in the gas sensing process. After air is injected, many oxygen molecules will adsorb on the material surface and oxygen vacancy sites via trapping the free electrons from the conduction band. Upon exposure to NO
2 gas, on account of the stronger electron affinity of NO
2 molecules, much more electrons will extract by NO
2, which increase the electrical resistance. Due to the excellent conductivity and high carrier mobility, the electron transfer in the material can proceed facilely, which greatly enhance the response and recovery rates.