Microfabrication of Embedding a Flexible Parylene-Based Microelectrode Array within Body-on-a-Chip †
Abstract
:1. Introduction
2. Materials and Methods
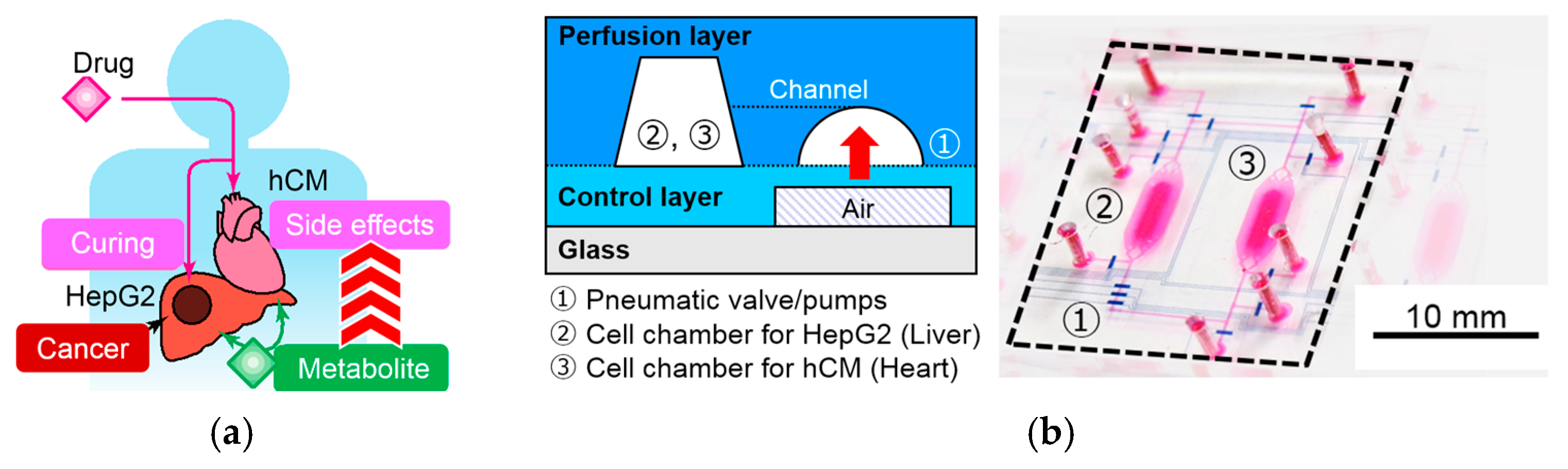
2.1. Device Design
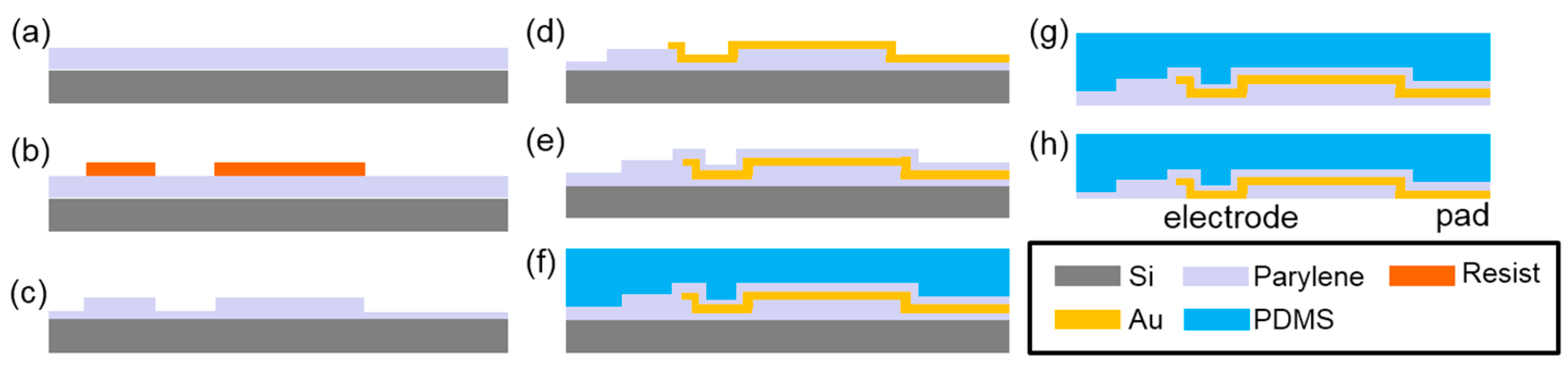
2.2. MEA Fabrication
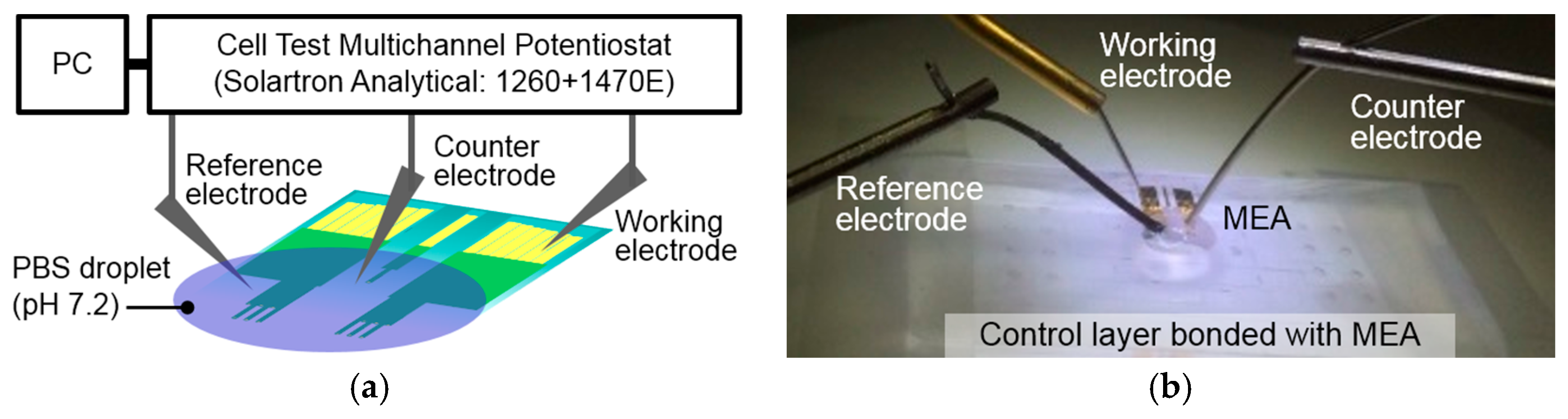
2.3. MEA Characterization
3. Results and Discussion
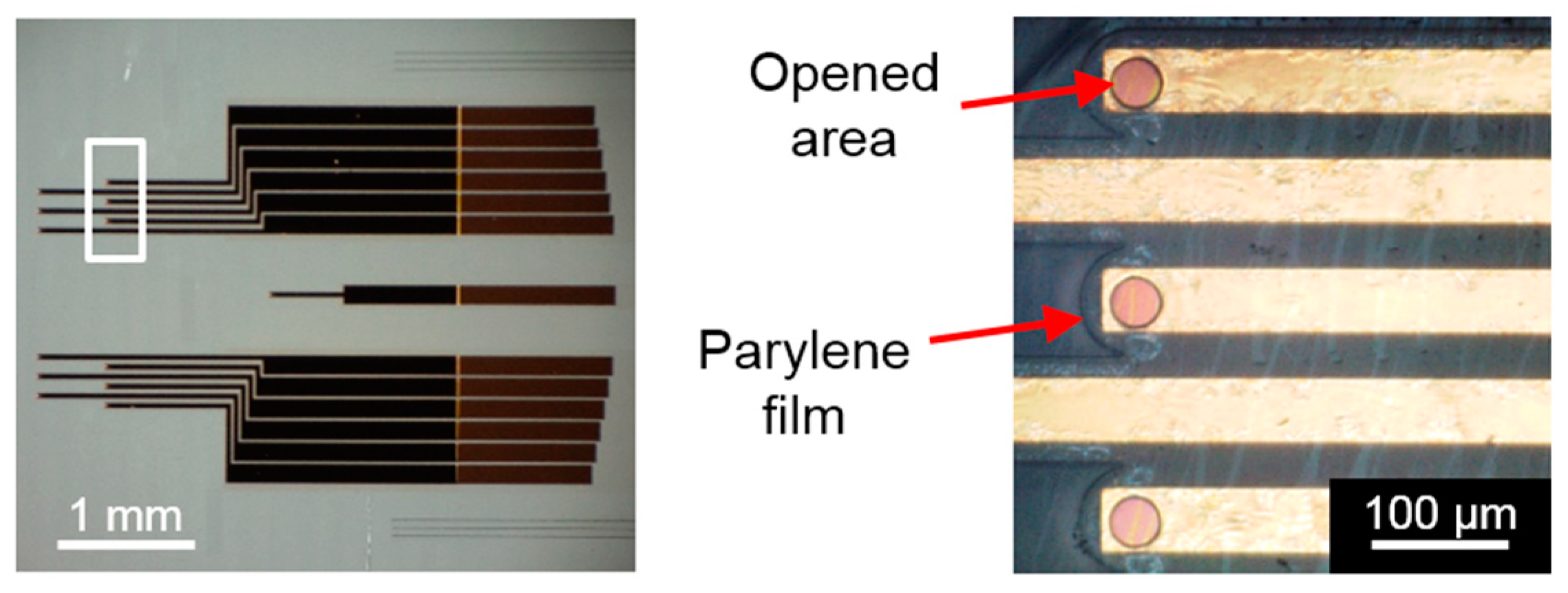
3.1. Device Fabrication
3.2. Electrical Properties
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hirai, Y.; Kamei, K.; Tsuchiya, T.; Tabata, O. Microfluidic device to interconnect multiple organs via fluidic circulation: Towards Body-on-a-Chip. In Proceedings of the 18th International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers 2015), Anchorage, AK, USA, 21–25 June 2015; pp. 1549–1552. [Google Scholar]
- Maoz, B.M.; Herland, A.; Henry, O.Y.F.; Leineweber, W.D.; Yadid, M.; Doyle, J.; Mannix, R.; Kujala, V.J.; FitzGerald, E.A.; Parker, K.K.; et al. Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 2017, 7, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Adrega, T.; Lacour, S.P. Stretchable gold conductors embedded in PDMS and patterned by photolithography: Fabrication and electromechanical characterization. J. Micromech. Microeng. 2010, 20, 055025. [Google Scholar] [CrossRef]
- Byun, I.; Coleman, A.W.; Kim, B. Transfer of thin Au films to polydimethylsiloxane (PDMS) with reliable bonding using (3-mercaptopropyl)trimethoxysilane (MPTMS) as a molecular adhesive. J. Micromech. Microeng. 2013, 23, 085016. [Google Scholar] [CrossRef]
- Pakazad, S.K.; Savov, A.; van de Stolpe, A.; Dekker, R. A novel stretchable micro-electrode array (SMEA) design for directional stretching of cells. J. Micromech. Microeng. 2014, 24, 034003. [Google Scholar] [CrossRef]
- Chang, T.Y.; Yadav, V.G.; Leo, S.D.; Mohedas, A.; Rajalingam, B.; Chen, C.L.; Selvarasah, S.; Dokmeci, M.R.; Khademhosseini, A. Cell and protein compatibility of Parylene-C surfaces. Langmuir 2007, 23, 11718–11725. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omaki, T.; Hirai, Y.; Kamei, K.-i.; Tsuchiya, T.; Tabata, O. Microfabrication of Embedding a Flexible Parylene-Based Microelectrode Array within Body-on-a-Chip. Proceedings 2017, 1, 302. https://doi.org/10.3390/proceedings1040302
Omaki T, Hirai Y, Kamei K-i, Tsuchiya T, Tabata O. Microfabrication of Embedding a Flexible Parylene-Based Microelectrode Array within Body-on-a-Chip. Proceedings. 2017; 1(4):302. https://doi.org/10.3390/proceedings1040302
Chicago/Turabian StyleOmaki, Tatsuya, Yoshikazu Hirai, Ken-ichiro Kamei, Toshiyuki Tsuchiya, and Osamu Tabata. 2017. "Microfabrication of Embedding a Flexible Parylene-Based Microelectrode Array within Body-on-a-Chip" Proceedings 1, no. 4: 302. https://doi.org/10.3390/proceedings1040302
APA StyleOmaki, T., Hirai, Y., Kamei, K.-i., Tsuchiya, T., & Tabata, O. (2017). Microfabrication of Embedding a Flexible Parylene-Based Microelectrode Array within Body-on-a-Chip. Proceedings, 1(4), 302. https://doi.org/10.3390/proceedings1040302





