Abstract
Two novel dicyanovinyl derivatives 3a–b were synthesized in moderate to good yields through a Knoevenagel reaction of the corresponding aldehyde precursors and malononitrile. The photophysical properties of the new push-pull systems were studied by UV-vis and fluorescence spectroscopy in acetonitrile. The evaluation of the compounds as colorimetric chemosensors was carried out by performing spectrophotometric titrations in acetonitrile and acetonitrile/water in the presence of relevant organic and inorganic anions, and of alkaline, alkaline-earth and transition metal cations. The benzoindole derivative exhibited great selectivity for the cyanide anion over other anions in acetonitrile/water (8:2) solution showing a distinct color change from colorless to yellow.
1. Introduction
The cyanide anion is well known due to its toxicity to the environment and to mammals, leading to convulsions, loss of consciousness, and eventual death. It is lethal to humans in concentrations in the range of 0.5–3.5 mg per Kg of body weight. In addition to being found in many foods and plants, cyanides are used industrially in the synthesis of organic chemicals, polymers, metallurgy as well as in gold mining [1,2,3,4].
Consequently, selective detection and quantification of cyanide is very important and it has been the object of increasing investigation. A large number of fluorimetric and/or colorimetric chemosensors as well as dosimeters, capable of detecting this anion in organic solvents as well as in aqueous mixtures have been reported during the last decade. Even so, the majority suffer from several drawbacks such as difficult synthesis, poor selectivity, only work in an organic media and the use of instrumentation is required [5]. Therefore, the research on versatile and tunable chemosensors capable of selective and sensitive colorimetric sensing of the cyanide anion, especially in mixed aqueous solutions, is still a challenge [6,7,8,9,10,11].
Having in mind the work developed in our research group concerning push-pull dicyanovinyl derivatives for several optical applications (SHG and TPA NLOphores) [12,13,14,15], we report in this work, the synthesis, characterization and evaluation of the photophysical properties and the chemosensory ability of novel optical chemosensors based on benzofuran and benzoindole systems functionalized with the dicyanovinyl group.
2. Experimental
2.1. General Procedure for the Synthesis of Dicyanovinyl Derivatives 3a–b
A solution of aldehyde 2a or 2b (0.37 mmol), malononitrile 1 (0.37 mmol) and piperidine (1 drop) in ethanol (5 mL) was heated at reflux for 5 h. After this time the solvent was evaporated and the resulting crude products were purified by column chromatography (silica gel, petroleum ether/dichloromethane (1:1)).
2.1.1. 2-((1H-Benzo[g]indol-3- yl)methylene)malononitrile 3a
Compound 3a was obtained as a light yellow solid (40 mg, 65%). Mp > 300 °C. UV-vis (acetonitrile): λmax nm (log ε) 374 (4.40). 1H-NMR (DMSO-d6): δ = 7.52 (dt, J = 8.0 and 1.2 Hz, 1H, H-7), 7.64 (dt, J = 8.0 and 1.2 Hz, 1H, H-8), 7.74 (d, J = 8.8 Hz, 1H, H-5), 8.0 (d, J = 8.0 Hz, 1H, H-6), 8.11 (d, J = 8.8 Hz, 1H, H-4), 8.41 (d, J = 8.4 Hz, 1H, H-9), 8.49 (s, 1H, H-2), 8.82 (s, 1H, CH=C), 13.55 (s, 1H, NH) ppm. 13C-NMR (DMSO-d6): δ = 70.91 (C-(CN)2), 112.38 (C-3a), 115.64 (C≡N), 115.71 (C≡N), 118.18 (C-4), 120.76 (C-9), 121.61 (C-9a), 123.28 (C-5), 123.39 (C-3), 125.14 (C-7), 126.57 (C-8), 128.62 (C-6), 129.91 (C-2), 130.70 (C-5a), 131.17 (C-3b), 153.05 (CH=C) ppm.
2.1.2. 2-((Benzofuran-2-yl)methylene)malononitrile 3b
Compound 3b was obtained as a yellow solid (49 mg, 74%). Mp 170.9–171.5 °C. UV-vis (acetonitrile): λmax nm (log ε) 361 (4.61). 1H-NMR (DMSO-d6): δ = 7.40 (dt, J = 8.0 and 1.2 Hz, 1H, H-5), 7.60 (dt, J = 8.4 and 1.2 Hz, 1H, H-6), 7.70 (dd, J = 8.8 and 0.8 Hz, 1H, H-7), 7.83 (s, 1H, H-3), 7.88 (dd, J = 8.0 and 1.2 Hz, 1H, H-4), 8.48 (s, 1H, CH=C) ppm. 13C-NMR (DMSO-d6): δ = 78.67 (C-(CN)2), 111.99 (C-7), 113.07 (C≡N), 114.35 (C≡N), 121.34 (C-3), 123.92 (C-4), 124.70 (C-5), 127.09 (C-3a), 130.25 (C-6), 145.44 (CH=C), 148.98 (C-2), 156.14 (C-7a) ppm.
2.2. Spectrophotometric Titrations of Compounds 3a–b
UV–visible absorption spectra (250–650 nm) were obtained using a Shimadzu UV/2501PC spectrophotometer. Fluorescence spectra were collected using a FluoroMax-4 spectrofluorometer. The relative fluorescence quantum yields were determined by using 10−6 M solution of Rhodamine 6G in ethanol as standard (ФF = 0.95) [16]. Organic solvents used in the spectroscopic studies were of spectroscopic grade. Solutions of derivatives 3a–b (ca. 1.0 × 10−5 M) and of the ions under study (ca. 1.0 × 10−2 and 1.0 × 10−3 M) were prepared in UV-grade acetonitrile or acetonitrile/water (8:2). Titrations of the compounds 3a–b in the presence of relevant organic and inorganic anions (AcO−, F−, Cl−, Br−, CN−, NO3−, BzO−, H2PO4−, HSO4−), and of alkaline, alkaline-earth and transition metal cations (Cu2+, Cd2+, Pd2+, Ni2+, Hg2+, Zn2+, Fe2+, Fe3+ and Cr3+) was performed by the sequential addition of the ion stock solution to the dicyanovinyl solution, in a 10 mm path length quartz cuvette and absorption emission spectra were measured by excitation at the wavelength of maximum absorption for each compound, with a 2 nm slit.
3. Results and Discussion
3.1. Synthesis and Characterization
Dicyanovinyl 3a–b were synthesized in moderate to good yields (65%–74%), by a Knoevenagel reaction between malononitrile 1 and aldehydes 2a–b. The new compounds were completely characterized by the usual spectroscopic techniques (Scheme 1, Table 1).
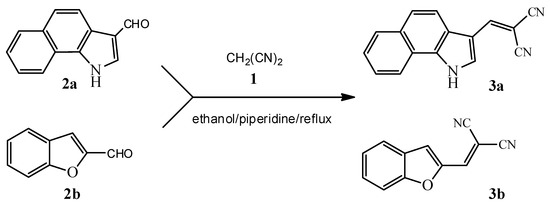
Scheme 1.
Synthesis of dicyanovinyl derivatives 3a–b.

Table 1.
Yields, UV-visible absorption and emission data for dicyanovinyls 3a–b in acetonitrile solution.
The absorption and emission spectra of compounds 3a–b were measured in acetonitrile solutions and showed intense lowest energy charge-transfer absorption bands in the UV-visible region between 361 and 374 nm (absorption). Both compounds were very weakly fluorescent, with wavelengths of maximum emission close to 405–528 nm and with relative fluorescence quantum yields in the order of 0.005.
3.2. Spectrophotometric Titrations of Dicyanovinyl Derivatives 3a–b with Anions and Metallic Ions
Evaluation of new dicyanovinyl 3a–b (10−5 M) as colorimetric chemosensors were carried out in ACN and ACN/H2O solutions, in the presence of several ions (AcO−, F−, Cl−, Br−, CN−, NO3−, BzO−, H2PO4−, HSO4−, Cu2+, Cd2+, Pd2+, Ni2+, Hg2+, Zn2+, Fe2+, Fe3+ and Cr3+) with biological, environmental and analytical relevance.
Preliminary tests were carried out by addition of up to 50 equiv of each ion to the solutions of dicyanovinyls 3a–b in ACN and in aqueous mixture ACN/H2O (8:2), revealing that only compound 3a exhibited chemosensor ability.
It was found that 3a displayed a marked colour change, from colourless to yellow, upon interaction with AcO, F−, CN−, BzO− and H2PO4− in acetonitrile solution. Moreover, 3a showed selectivity for the cyanide ion in aqueous mixture displaying the same colour change (Figure 1).
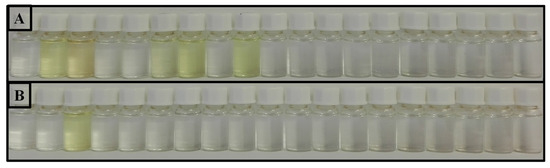
Figure 1.
Colour changes of compound 3a (10−4 M in (A): ACN, (B): ACN/H2O (8:2)) in the presence of 50 equiv. of AcO−, CN−, HSO4−, NO3−, H2PO4−, F−, Cl−, BzO−, Cu2+, Pd2+, Zn2+, Fe2+, Hg2+, Fe3+, Co2+, Ca2+, Na+ and Ni2+ (in the form of tetrafluorborate or perchlorate salts).
Considering these preliminary results, spectrophotometric titration of compound 3a in ACN with these selected ions were undertaken. Titration with CN− revealed a trend in the UV-Vis spectra, the intensity of the longest wavelength absorption band at 373 nm decreased progressively upon addition of the anion, with the simultaneous growth of a new red-shifted absorption band located at 424 nm (Figure 2). It can be seen that a very small amount of cyanide ion (0.3 equiv) caused large changes in the absorption spectrum and a drastic color change in the solution of compound 3a.
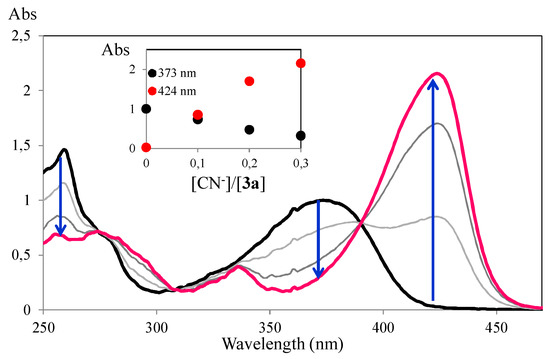
Figure 2.
Spectrophotometric titrations of compound 3a with addition of increasing amounts of CN− in ACN. The inset represents the normalized absorption ([3a] = 1 × 10−5 M, T = 298 K).
Compound 3a, in the titration with F−, AcO−, H2PO4− and BzO−, revealed the same trend observed in the titration with cyanide ion (Figure 3). In the titration with F− 0.4 equiv were used, 1 equiv was needed for titration with AcO−, and 5 equiv for the titration with H2PO4− and BzO−. Thus, although not selective, compound 3a in acetonitrile was more sensitive to the presence of cyanide ion, considering that only 0.3 equiv of the cyanide ion were required for the visible colour change.
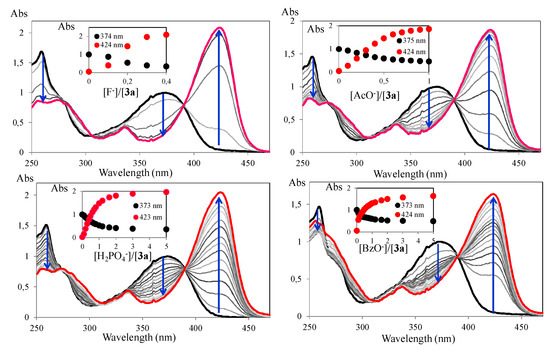
Figure 3.
Spectrophotometric titration of 3a with addition of increasing amounts of F−, AcO−, H2PO4− and BzO− in ACN. The inset represents the normalized absorption a 373 nm ([3a] = 1 × 10−5 M, T = 298 K).
Spectrophotometric titrations of compound 3a in ACN/H2O (8:2) confirmed the preliminary sensing results, with compound 3a being selective for the cyanide ion although it required a larger amount of ion to achieve a similar colour change (70 equiv), when compared to the titration in ACN. In Figure 4 it can be seen that there was a gradual decrease in absorption intensity upon addition of the cyanide ion, accompanied by a red-shift with the formation of a new band at 417 nm.
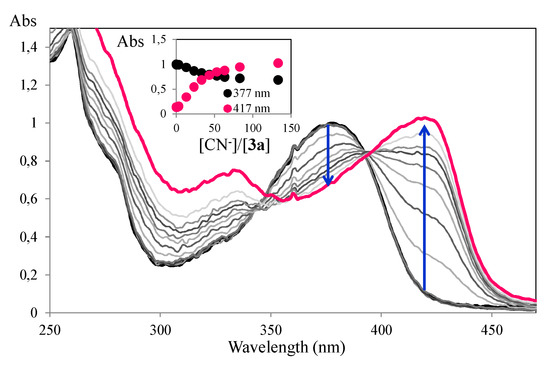
Figure 4.
Spectrophotometric titration of compound 3a with addition of increasing amounts of CN− in ACN/H2O (8:2). The inset represents the normalized emission ([3a] = 1 × 10−5 M, T = 298 K).
4. Conclusions
The synthesis of new dicyanovinyls 3a–b was achieved in moderate to good yields by a simple experimental procedure. The sensory ability was evaluated for several ions by spectrophotometric titrations in acetonitrile and acetonitrile/water. Compound 3a was selective for the cyanide ion in ACN/H2O (8:2), which is a very promising result as a colorimetric chemosensor for application in aqueous media.
Acknowledgments
Thank are due to Fundação para a Ciência e Tecnologia (Portugal) and FEDER-COMPETE for financial support through Centro de Química (UID/QUI/00686/2013 and UID/QUI/0686/2016), and a PhD grant to R.C.M. Ferreira (SFRH/BD/86408/2012). The NMR spectrometer Bruker Avance III 400 is part of the National NMR Network and was purchased with funds from FCT and FEDER.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vennesland, B.; Comm, E.E.; Knownles, C.J.; Westly, J.; Wissing, F. Cyanide in Biology; Academic Press: London, UK, 1981. [Google Scholar]
- Bohnet, M. (Ed.) Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: New York, NY, USA, 2003. [Google Scholar]
- Muir, G. Hazards in the Chemical Laboratory; Royal Chemical Society: London, UK, 1977. [Google Scholar]
- Baskin, S.I.; Brewer, T.G. Medical Aspects of Chemical and Biological Warfare; Sidel, F., Takafuji, E.T., Franz, D.R., Eds.; TMM Publications: Washington, DC, USA, 1997; p. 271. [Google Scholar]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M. Selective colorimetric and fluorimetric detection of cyanide in aqueous solution using novel heterocyclic imidazo-anthraquinones. Sens. Actuators B Chem. 2014, 191, 791. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Oliveira, E.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Cyanide and fluoride colorimetric sensing by novel imidazo-anthraquinones functionalized with indole and carbazole. Supramol. Chem. 2014, 26, 71. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M. J. Photochem. Photobiol. Chem. A 2013, 259, 733.
- Santos-Figueroa, L.E.; Moragues, M.E.; Raposo, M.M.M.; Batista, R.M.F.; Ferreira, R.C.M.; Costa, S.P.G.; Sancenón, F.; Martínez-Máñez, R.; Ros-Lis, J.V.; Soto, J. Synthesis and evaluation of thiosemicarbazones functionalized with furyl moieties as new chemosensors for anion recognition. Org. Biomol. Chem. 2012, 10, 7418. [Google Scholar] [CrossRef] [PubMed]
- Santos-Figueroa, L.E.; Moragues, M.E.; Raposo, M.M.M.; Batista, R.M.F.; Ferreira, R.C.M.; Costa, S.P.G.; Sancenón, F.; Martínez-Máñez, R.; Ros-Lis, J.V.; Soto, J. Synthesis and evaluation of fluorimetric and colorimetric chemosensors for anions based on (oligo)thienyl-thiosemicarbazones. Tetrahedron 2012, 68, 7179. [Google Scholar] [CrossRef]
- Raposo, M.M.M.; García-Acosta, B.; Ábalos, T.; Calero, P; Martínez-Manez, R.; Ros-Lis, J.V.; Soto, J. Synthesis and study of the use of heterocyclic thiosemicarbazones as signalling scaffolding for the recognition of anions. J. Org. Chem. 2010, 75, 2922. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.M.M.; Herbivo, C.; Hugues, V.; Clermont, G.; Castro, M.C.R.; Comel, A.; Blanchard-Desce, M. Synthesis, fluorescence and two-photon absorption properties of push-pull 5-aryl[3,2-b]thienothiophene derivatives. Eur. J. Org. Chem. 2016, 31, 5263–5273. [Google Scholar]
- Castro, M.C.R.; Belsley, M.; Raposo, M.M.M. Push-pull second harmonic generation (SHG) chromophores bearing pyrrole and thiazole heterocycles functionalized with strong acceptor moieties: syntheses and characterization. Dyes Pigments 2016, 128, 89–95. [Google Scholar] [CrossRef]
- Castro, M.C.R.; Belsley, M.; Raposo, M.M.M. Synthesis and characterization of push-pull (bi)thienylpyrrole NLOphores with enhanced hyperpolarizabilities. Dyes Pigments 2016, 131, 333–335. [Google Scholar] [CrossRef]
- Genin, E.; Hugues, V.; Clermont, G.; Herbivo, C.; Comel, A.; Castro, M.C.R.; Raposo, M.M.M.; Blanchard-Desce, M. Fluorescence and two-photon absorption of push-pull aryl(bi)thiophenes: structure-property relationships. Photochem. Photobiol. Sci. 2012, 11, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Kubin, R.F.; Fletcher, A.N. Fluorescence quantum yields of some rhodamine dyes. J. Lumin. 1982, 27, 455–462. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).