Rhizobacteriome Diversity and Morphophysiological Features of Three Tomato Plant Varieties Under Nitrogen Deficiency
Abstract
1. Introduction
2. Materials and Methods
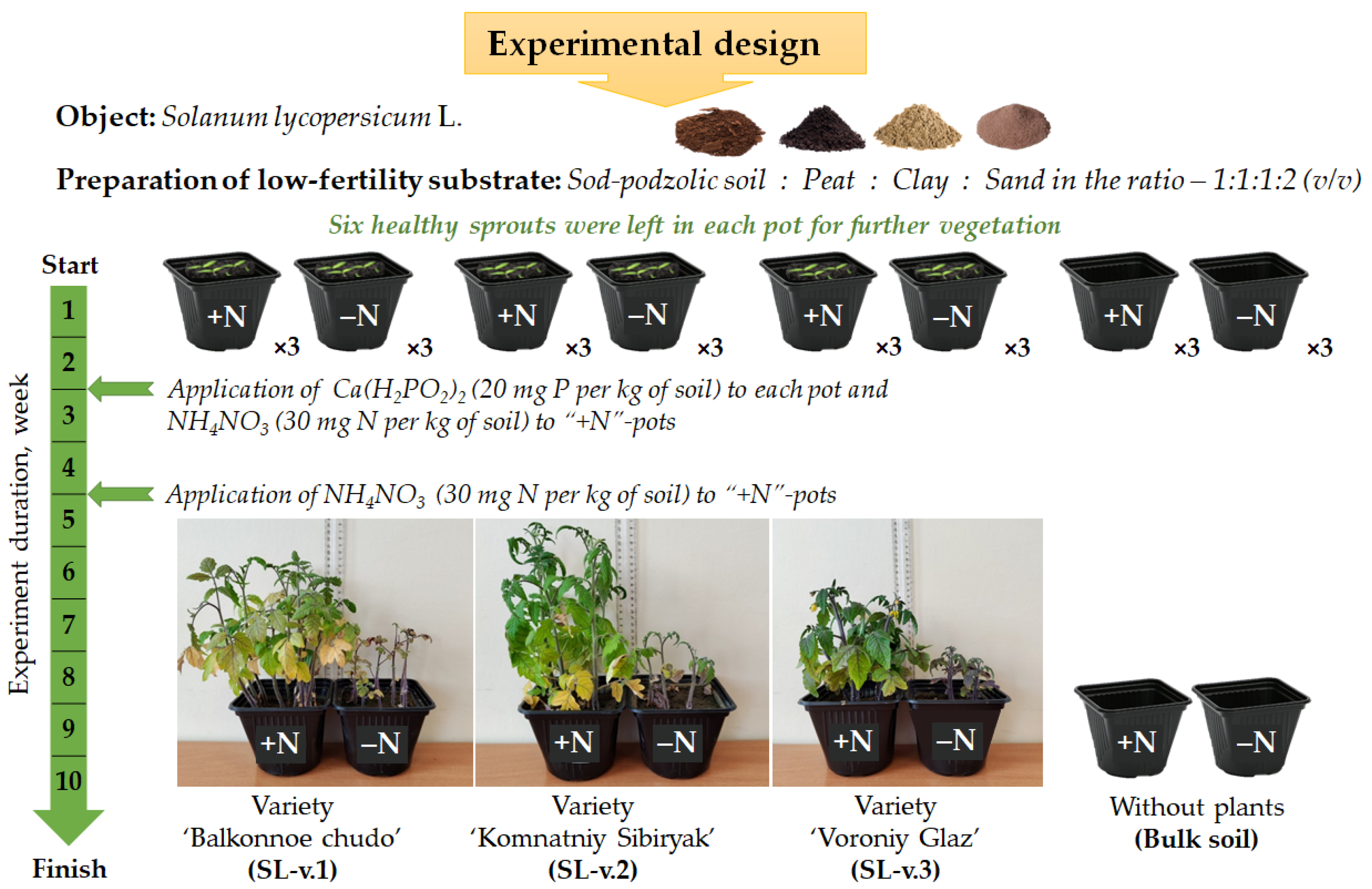
2.1. Plant Material and Experimental Design
2.2. Soil DNA Extraction, Nanopore Sequencing, and Bioinformatic Analysis
2.3. Isolation and Enumeration of Microorganisms, Including N-Cycling Rhizobacteria
2.4. Estimation of Plant Morphometric and Physiological Characteristics
2.5. Assessment of pH, Different N Forms in Plant and Soil Samples
2.6. Statistical Analysis
3. Results
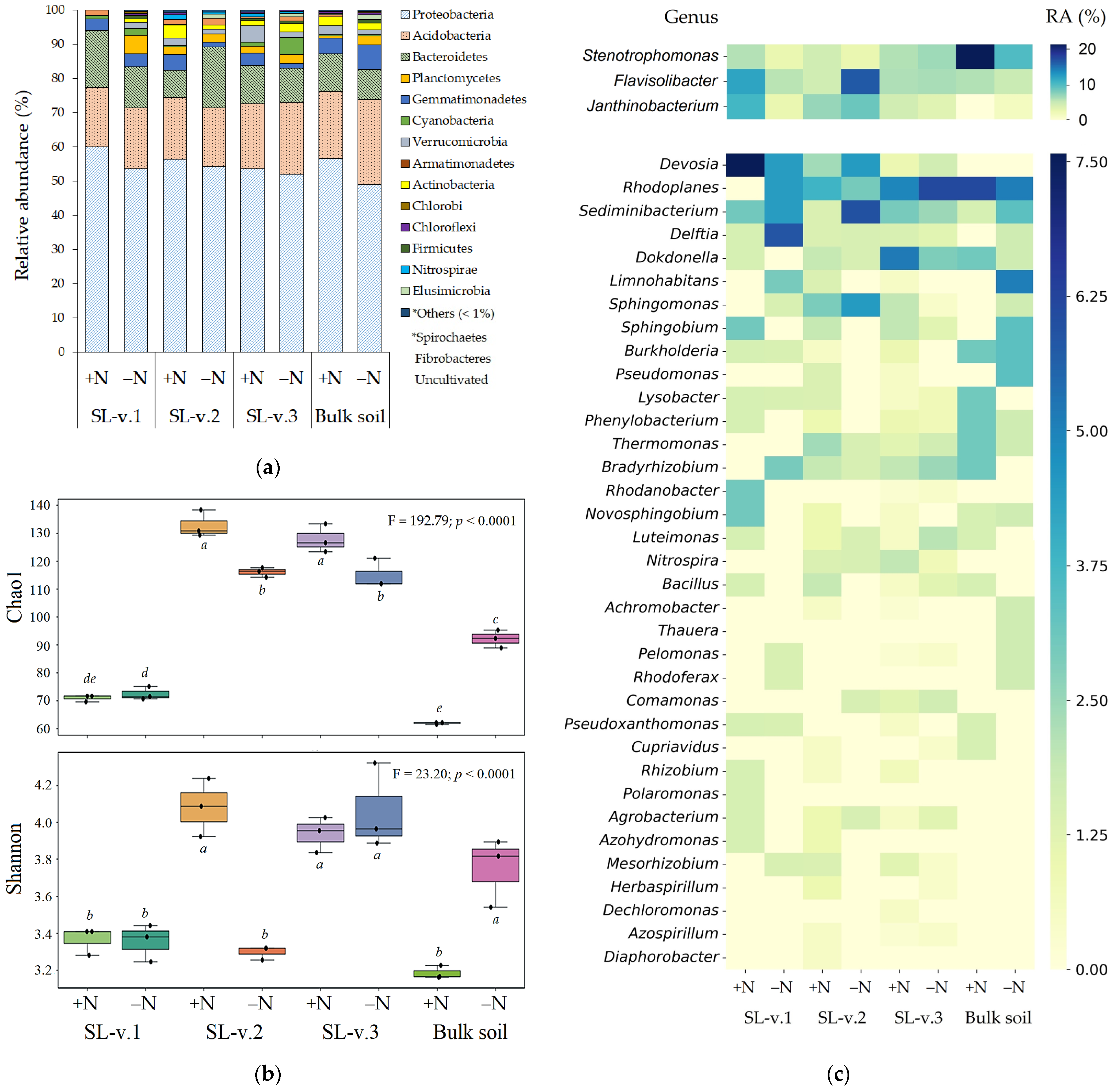
3.1. Rhizobacteriome Diversity and Composition
3.2. Total Microbial Counts and N-Cycling Rhizobacteria
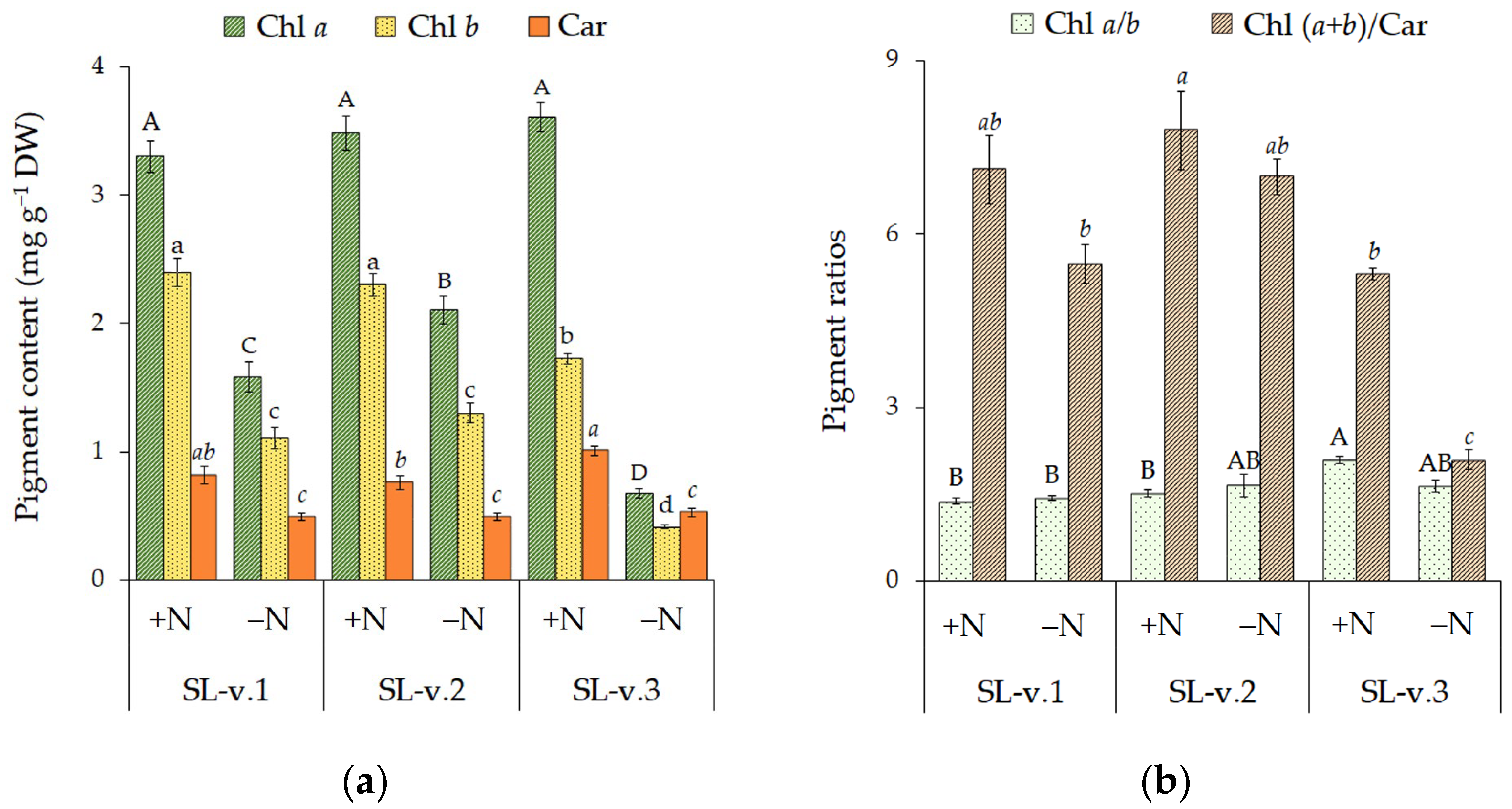
3.3. Plant Morphometric and Physiological Characteristics
3.4. Fine Root Structural Characteristics
3.5. Nitrogen Content in Plant Biomass
3.6. Different N Forms and pH Values in Substrate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADS | Air-dry substrate |
| Nalk.h. | Alkaline-hydrolyzable nitrogen |
| NH4+ | Ammonium cations |
| NH4NO3 | Ammonium nitrate |
| N–NH4+ | Ammonium nitrogen |
| ANOVA | Analysis of variance |
| CFU | Colony-forming units |
| Car | Carotenoids |
| Chl | Chlorophyll |
| DW | Dry weight |
| FW | Fresh weight |
| LB medium | Luria–Bertani medium |
| Ca(H2PO4)2 | Monocalcium phosphate |
| MPN | Most probable number |
| N | Nitrogen |
| NO3− | Nitrate anions |
| N-NO3− | Nitrate nitrogen |
| QMAFAnM | Quantity of mesophilic aerobic and facultative anaerobic microorganisms |
| RA | Relative abundance |
| RDA | Redundancy Analysis |
| SE | Standard error |
| SL-v.1 | Solanum lycopersicum variety ‘Balkonnoe Chudo’ |
| SL-v.2 | Solanum lycopersicum variety ‘Komnatniy Sibiryak’ |
| SL-v.3 | Solanum lycopersicum variety ‘Voroniy Glaz’ |
| Ntot. | Total amount of nitrogen |
Appendix A
Appendix A.1
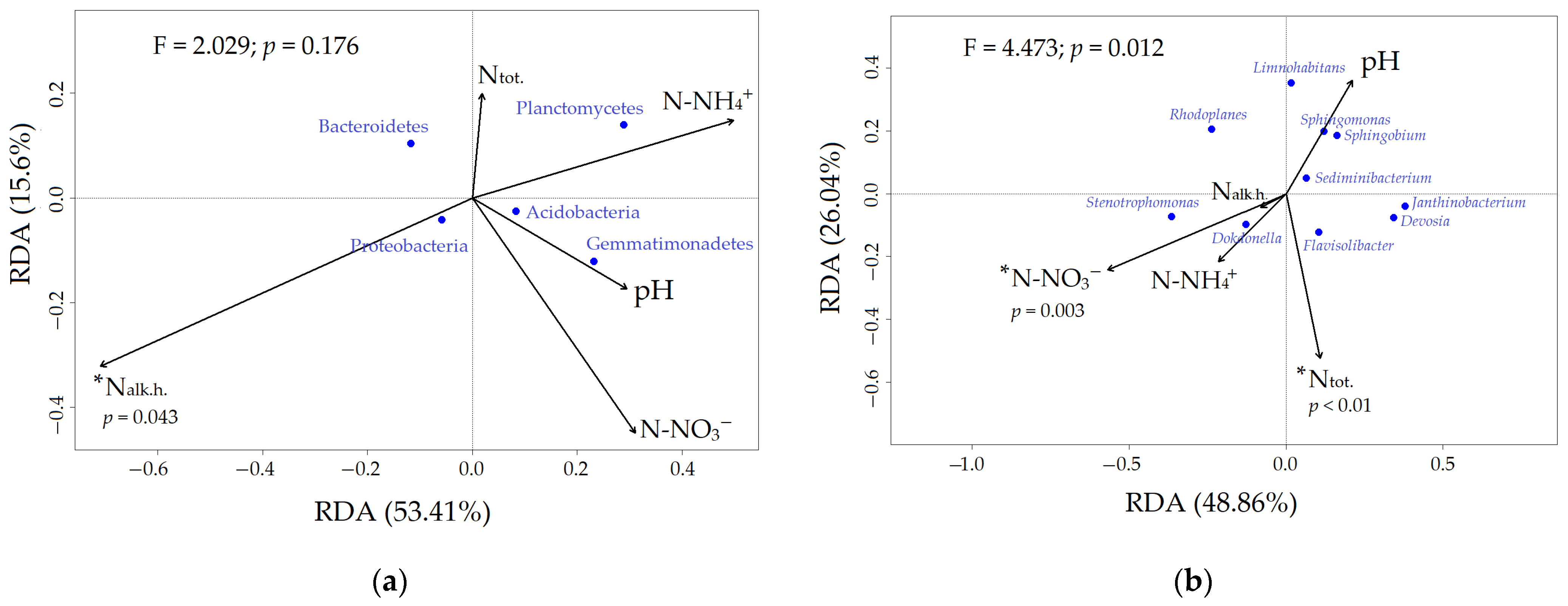
Appendix A.2
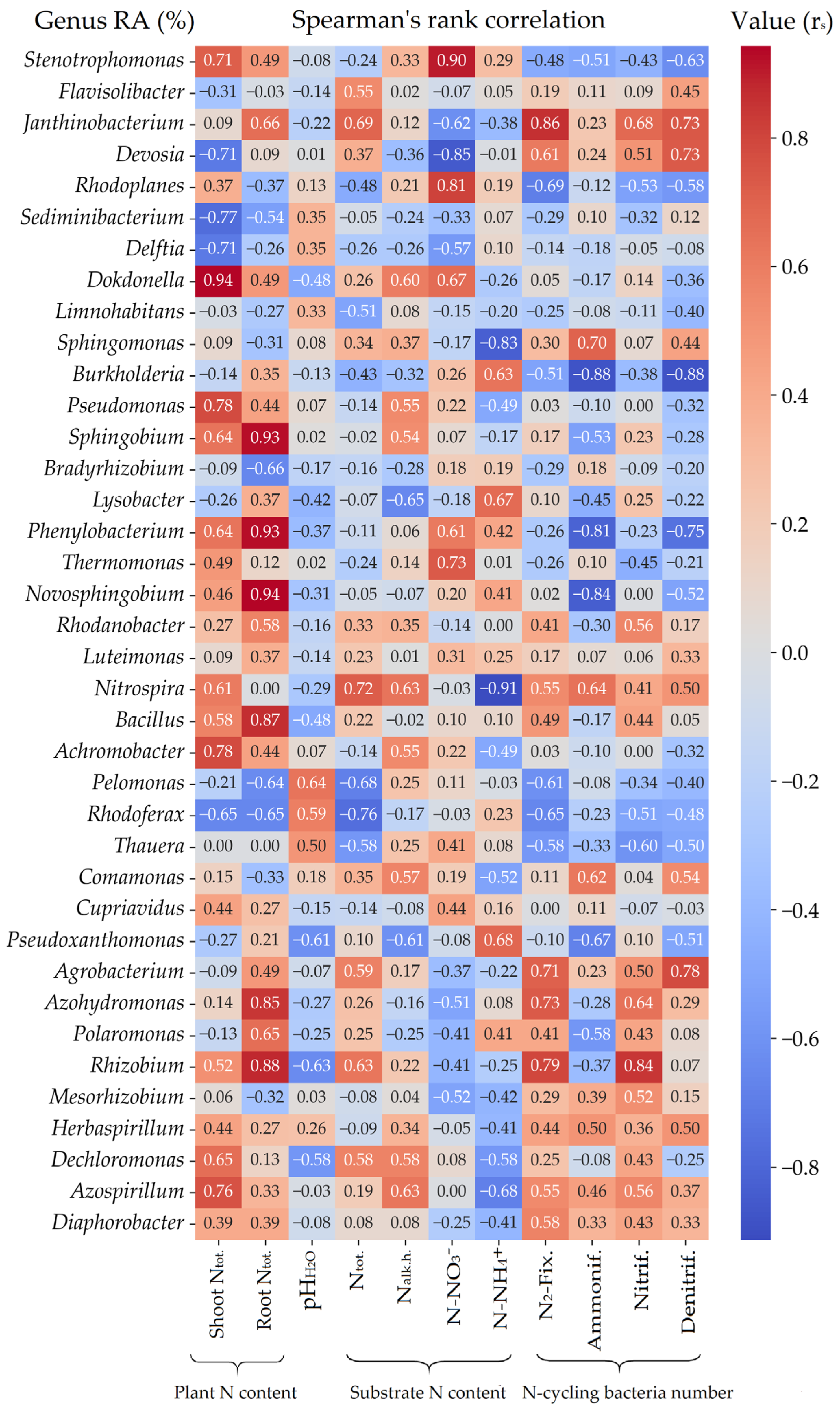
Appendix A.3
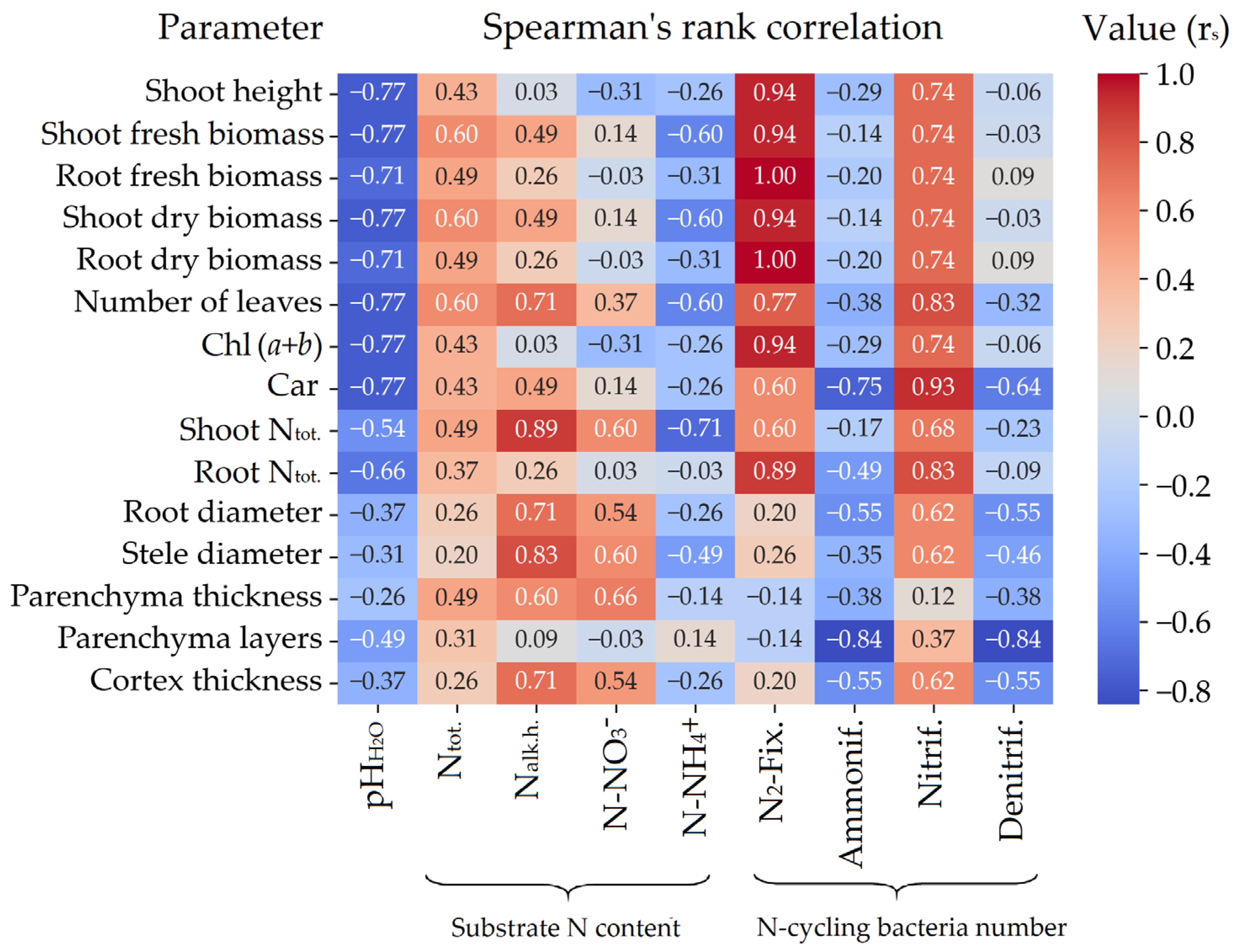
References
- Filippova, L.S. Nitrogen cycling and nitrogen compounds in the soil. Int. Res. J. 2023, 8, 134. Available online: https://research-journal.org/archive/8-134-2023-august/10.23670/IRJ.2023.134.37 (accessed on 7 November 2025). (In Russian).
- Lynch, J.P.; Galindo-Castañeda, T.; Schneider, H.M.; Sidhu, J.S.; Rangarajan, H.; York, L.M. Root phenotypes for improved nitrogen capture. Plant Soil 2024, 502, 31–85. [Google Scholar] [CrossRef]
- Rosado, S.I.P.; Santos, J.Z.L.; Saraiva, I.S.D.A.; dos Santos, N.J.R.; Barbosa, T.M.B.; Araujo, J.L. Nitrate/ammonium ratios and nitrogen deficiency impact on nutrient absorption and photosynthetic efficiency of Cedrela odorata. Nitrogen 2024, 5, 1–15. [Google Scholar] [CrossRef]
- Mejia, G.; Jara-Servin, A.; Hernández-Álvarez, C.; Romero-Chora, L.; Peimbert, M.; Cruz-Ortega, R.; Alcaraz, L.D. Rhizosphere microbiome influence on tomato growth under low–Nutrient settings. FEMS Microbiol. Ecol. 2025, 101, fiaf019. [Google Scholar] [CrossRef]
- Muratova, A.; Hübner, T.; Narula, N.; Wand, H.; Turkovskaya, O.; Kuschk, P.; Jahn, R.; Merbach, W. Rhizosphere microflora of plants used for the phytoremediation of bitumen-contaminated soil. Microbiol. Res. 2003, 158, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Hannah, K.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, T.; Li, Y.; Zheng, X. A critical review of the central role of microbial regulation in the nitrogen biogeochemical process: New insights for controlling groundwater nitrogen contamination. J. Environ. Manag. 2023, 328, 116959. [Google Scholar] [CrossRef]
- Galindo-Castañeda, T.; Lynch, J.P.; Six, J.; Hartmann, M. Improving soil resource uptake by plants through capitalizing on synergies between root architecture and anatomy and root-associated microorganisms. Front. Plant Sci. 2022, 13, 827369. [Google Scholar] [CrossRef]
- Boyarshina, K.S.; Adamova, V.V.; Zhenga, W.; Nikitinskaya, E.V.; Obukhova, O.Y.; Kolkova, M.V.; Nesterenko, V.A.; Bespalova, O.S.; Klyueva, V.V.; Degtyareva, K.A.; et al. Dominant bacterial taxa in chernozems and factors affecting their abundance in the bacterial community. Eurasian Soil Sci. 2024, 57, 1007–1017. [Google Scholar] [CrossRef]
- González, M.; Araya-Angel, J.P.; Muñoz, A.; Alfaro-Flores, A.; Cardinale, M.; Stoll, A. Genetic diversification of tomato and agricultural soil management shaped the rhizospheric microbiome of tomato (Solanum lycopersicum). Microorganisms 2025, 13, 1550. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Strauss, S.L.; González-López, J.; Bedmar, E.J. Changes in the diversity and predicted functional composition of the bulk and rhizosphere soil bacterial microbiomes of tomato and common bean after inorganic N-fertilization. Rhizosphere 2021, 18, 100362. [Google Scholar] [CrossRef]
- Kavamura, V.N.; Hayat, R.; Clark, I.M.; Rossmann, M.; Mendes, R.; Hirsch, P.R.; Mauchline, T.H. Inorganic nitrogen application affects both taxonomical and predicted functional structure of wheat rhizosphere bacterial communities. Front. Microbiol. 2018, 29, 1074. [Google Scholar]
- Muhammad, I.; Yang, L.; Ahmad, S.; Zeeshan, M.; Farooq, S.; Ali, I.; Khan, A.; Zhou, X.B. Irrigation and nitrogen fertilization alter soil bacterial communities, soil enzyme activities, and nutrient availability in maize crop. Front. Microbiol. 2022, 13, 833758. [Google Scholar] [CrossRef]
- Chen, J.; Arafat, Y.; Ud Din, I.; Yang, B.; Zhou, L.; Wang, J.; Letuma, P.; Wu, H.; Qin, X.; Wu, L.; et al. Nitrogen fertilizer amendment alter the bacterial community structure in the rhizosphere of rice (Oryza sativa L.) and improve crop yield. Front. Microbiol. 2019, 10, 2623. [Google Scholar] [CrossRef]
- Amare, G. Review on mineral nutrition of onion (Allium cepa L.). Open Biotechnol. J. 2020, 14, 134–144. [Google Scholar] [CrossRef]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sighu, J.S.; Ajmera, I.; Galindo-Castañeda, T.; Klein, S.P.; Hanlon, M.T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
- Marques, D.N.; Gaziola, S.A.; Piotto, F.A.; Azevedo, R.A. Phytochelatins: Advances in Tomato Research. Agronomy 2025, 15, 80. [Google Scholar] [CrossRef]
- Gatsios, A.; Ntatsi, G.; Yfantopoulos, D.; Baltzoi, P.; Karapanos, I.C.; Tsirogiannis, I.; Patakioutas, G.; Savvas, D. Effects of different organic soil amendments on nitrogen nutrition and yield of organic greenhouse tomato crop. Nitrogen 2021, 2, 347–358. [Google Scholar] [CrossRef]
- Naumova, N.; Baturina, O.; Nechaeva, T.; Kabilov, M. Root and rhizosphere microbiome of tomato plants grown in the open field in the south of West Siberia under mineral fertilization. Horticulturae 2022, 8, 1051. [Google Scholar] [CrossRef]
- Allard, S.M.; Walsh, C.S.; Wallis, A.E.; Ottesen, A.R.; Brown, E.W.; Micallef, S.A. Solanum lycopersicum (tomato) hosts robust phyllosphere and rhizosphere bacterial communities when grown in soil amended with various organic and synthetic fertilizers. Sci. Total Environ. 2016, 573, 555–563. [Google Scholar] [CrossRef]
- Dong, C.J.; Wang, L.L.; Li, Q.; Shang, Q.M. Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE 2019, 14, e0223847. [Google Scholar] [CrossRef]
- Cirillo, V.; Romano, I.; Woo, S.L.; Di Stasio, E.; Lombardi, N.; Comite, E.; Pepe, O.; Ventorino, V.; Maggio, A. Inoculation with a microbial consortium increases soil microbial diversity and improves agronomic traits of tomato under water and nitrogen deficiency. Front. Plant Sci. 2023, 14, 1304627. [Google Scholar] [CrossRef]
- Cochavi, A.; Cohen, I.H.; Rachmilevitch, S. The role of different root orders in nutrient uptake. Environ. Exp. Bot. 2020, 179, 104212. [Google Scholar] [CrossRef]
- Saha, S.; Huang, L.; Khoso, M.A.; Wu, H.; Han, D.; Ma, X.; Poudel, T.R.; Li, B.; Zhu, M.; Lan, Q.; et al. Fine root decomposition in forest ecosystems: An ecological perspective. Front. Plant Sci. 2023, 14, 1277510. [Google Scholar] [CrossRef]
- Chao, A. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Shannon, C.E. The mathematical theory of communication. 1963. MD Comput. 1997, 14, 306–317. [Google Scholar]
- Netrusov, A.I.; Egorova, M.A.; Zakharchuk, L.M.; Kolotilova, N.N. Practical Training in Microbiology: Textbook for Students of Higher Educational Institutions; Netrusov, A.I., Ed.; Publishing Center “Academy”: Moscow, Russia, 2005. (In Russian) [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Betekhtina, A.A.; Tukova, D.E.; Veselkin, D.V. Root structure syndromes of four families of monocots in the Middle Urals. Plant Diversity 2023, 45, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Betekhtina, A.A.; Nekrasova, O.A.; Malakheeva, A.V.; Voropaeva, O.V.; Maleva, M.G. Nitrogen and phosphorus content in cereals and dicot grasses leaves and features of their rhizosphere microflora in multi-age fly ash dumps. Biol. Bull. Russ. Acad. Sci. 2025, 52, 116. [Google Scholar] [CrossRef]
- Dodor, D.E.; Kamara, M.S.; Asamoah-Bediako, A.; Adiku, S.G.K.; MacCarthy, D.S.; Kumahor, S.K.; Neina, D. Evaluation of alkaline hydrolyzable organic nitrogen as an index of nitrogen mineralization potential of some coastal savannah soils of Ghana. Nitrogen 2022, 3, 652–662. [Google Scholar] [CrossRef]
- Tugbaeva, A.S.; Ermoshin, A.A.; Shiryaev, G.I.; Kiseleva, I.S. Microbiome of the soil and rhizosphere of the halophyte Spergularia marina (L.) Griseb in the Saline Sites of Lake Kurgi, the South Urals: Metagenomic Analysis. Microbiol. Res. 2025, 16, 64. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Fu, Y.; Zhao, H.; Cheng, J.; Xie, J.; Jiang, D. Enrichment of bacteria involved in the nitrogen cycle and plant growth promotion in soil by sclerotia of rice sheath blight fungus. Stress Biol. 2022, 2, 32. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, M.; Chen, Y.; Luo, J.; Hu, Y. Carbon selection for nitrogen degradation pathway by Stenotrophomonas maltophilia: Based on the balances of nitrogen, carbon and electron. Bioresour. Technol. 2019, 294, 122114. [Google Scholar] [CrossRef]
- Mikhailovich, V.; Heydarov, R.; Zimenkov, D.; Chebotar, I. Stenotrophomonas maltophilia virulence: A current view. Front. Microbiol. 2024, 15, 1385631. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lu, D.; Qin, B.; Liu, Q.; Zhao, Y.; Liu, H.; Ma, J. Highly efficient nitrogen removal of a coldness-resistant and low nutrient needed bacterium, Janthinobacterium sp. M-11. Bioresour. Technol. 2018, 256, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jin, P.; Cui, Z.; Xu, T.; Zhao, R.; Zheng, Z. Identification and characterization of Janthinobacterium svalbardensis F19, a novel low-C/N-tolerant denitrifying bacterium. Appl. Sci. 2019, 9, 1937. [Google Scholar] [CrossRef]
- Lindström, K.; Mousavi, S.A. Effectiveness of nitrogen fixation in rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335. [Google Scholar] [CrossRef]
- Naher, K.; Miwa, H.; Okazaki, S.; Yasuda, M. Effects of different sources of nitrogen on endophytic colonization of rice plants by Azospirillum sp. B510. Microbes Environ. 2018, 33, 301–308. [Google Scholar] [CrossRef]
- Hanson, B.T.; Yagi, J.M.; Jeon, C.O.; Madsen, E.M. Role of nitrogen fixation in the autecology of Polaromonas naphthalenivorans in contaminated sediments. Environ. Microbiol. 2012, 14, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Liu, B.; Braker, G. Isolation, genetic and functional characterization of novel soil nirK-type denitrifiers. Syst. Appl. Microbiol. 2010, 33, 337–347. [Google Scholar] [CrossRef]
- Wu, T.; Li, J.; Cao, R.; Chen, X.; Wang, B.; Huang, T.; Wen, G. Nitrate removal by a novel aerobic denitrifying Pelomonas puraquae WJ1 in oligotrophic condition: Performance and carbon source metabolism. Sci. Total Environ. 2024, 954, 176614. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.F.; Valk, L.C.; Verhoeven, M.D.; Nierychlo, M.A.; Singleton, C.M.; Dueholm, M.K.D.; Nielsen, P.H. Diversity and physiology of abundant Rhodoferax species in global wastewater treatment systems. Syst. Appl. Microbiol. 2025, 48, 126574. [Google Scholar] [CrossRef]
- Huo, C.; Zhang, J.; Yang, X.; Li, X.; Yu Su, Y.; Chen, Z. Dry season irrigation promotes nutrient cycling by reorganizing Eucalyptus rhizosphere microbiome. Sci. Total Environ. 2024, 954, 176307. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y.; Dong, Y.; Lapen, D.R.; Liu, J.; Chen, W. Subsoiling and conversion to conservation tillage enriched nitrogen cycling bacterial communities in sandy soils under long-term maize monoculture. Soil Tillage Res. 2022, 215, 105197. [Google Scholar] [CrossRef]
- Vijayan, A.; Jayadradhan, R.K.V.; Pillai, D.; Geetha, P.P.; Joseph, V.; Sarojini, B.S.I. Nitrospira as versatile nitrifiers: Taxonomy, ecophysiology, genome characteristics, growth, and metabolic diversity. J. Basic Microbiol. 2021, 61, 88–109. [Google Scholar] [CrossRef]
- Fowler, S.J.; Palomo, A.; Dechesne, A.; Mines, P.D.; Smets, B.F. Comammox Nitrospira are abundant ammonia oxidizers in diverse groundwater-fed rapid sand filter communities. Environ. Microbiol. 2018, 20, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Mawalagedera, S.M.M.R.; Weerakkody, W.A.P.; Premaratne, K.P. Circulation culture of tomato for efficient nutrient uptake and high yield in tropical greenhouses. Trop. Agric. Res. 2012, 23, 204–217. [Google Scholar] [CrossRef]
- Erabadupitiya, H.R.U.T.; Weerakkody, W.A.P.; Nandasena, K.A. Critical nitrogen ranges for growth stages of tomato in soilless culture under greenhouse conditions in the tropics. Int. J. Veg. Sci. 2022, 28, 25–39. [Google Scholar] [CrossRef]
- Maslennikova, D.R.; Chubukova, O.V.; Vershinina, Z.R.; Emelina, A.A.; Nasyrova, K.R.; Khakimova, L.R.; Mikhaylova, E.V. Effect of PGPR bacteria on growth and content of photosynthetic pigments in the leaves of tomato plants. Biomics 2021, 13, 274–279. (In Russian) [Google Scholar] [CrossRef]
- Lambers, H.; Albornoz, F.; Kotula, L.; Laliberté, E.; Ranathunge, K.; Teste, F.P.; Zemunik, G. How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyper-diverse ecosystems. Plant Soil 2018, 424, 11–33. [Google Scholar] [CrossRef]
- Salas-González, I.; Reyt, G.; Flis, P.; Custódio, V.; Gopaulchan, D.; Bakhoum, N.; Dew, T.P.; Suresh, K.; Franke, R.B.; Dangl, J.L.; et al. Coordination between microbiota and root endodermis supports plant mineral nutrient homeostasis. Science 2021, 371, eabd0695. [Google Scholar] [CrossRef] [PubMed]
- Borisova, G.G.; Voropaeva, O.V.; Maleva, M.G.; Kumar, A.; Tripti. Evaluation of the growth-promoting attributes of rhizo-bacteria Bacillus sp. and their influence on the morphophysiological characteristics of rapeseed. Agrar. Bull. 2023, 236, 2–13. [Google Scholar]


| Tomato Variety | Treatment | 1 QMAFAnM, ×107 CFU g−1 of Dry Soil | 1 N-Fixers, ×106 CFU g−1 of Dry Soil | 2 Ammonifiers, ×106 MPN g−1 of Dry Soil | 2 Nitrifiers, ×103 MPN g−1 of Dry Soil | 2 Denitrifiers, ×106 MPN g−1 of Dry Soil |
|---|---|---|---|---|---|---|
| SL-v.1 | +N | 2.409 ± 0.215 bc | 16.767 ± 0.731 a | 0.115 | 0.095–20.00 | 15.0 |
| –N | 1.646 ± 0.058 cd | 1.704 ± 0.007 b | 1.500 | 0.15–4.50 | 11.5 | |
| SL-v.2 | +N | 2.182 ± 0.141 bcd | 20.671 ± 1.702 a | 2.500 | 0.01–20.00 | 25.0 |
| –N | 1.469 ± 0.107 d | 3.346 ± 0.047 b | 4.500 | 0.01–1.50 | 45.0 | |
| SL-v.3 | +N | 4.896 ± 0.110 a | 4.766 ± 0.212 b | 0.950 | 0.095–20.00 | 9.5 |
| –N | 3.323 ± 0.125 b | 2.628 ± 0.022 b | 2.500 | 0.095–4.50 | 25.0 | |
| Bulk soil | +N | 2.446 ± 0.205 b | 0.683 ± 0.058 b | 0.75 | 0.045–0.95 | 1.5 |
| –N | 2.748 ± 0.199 b | 0.612 ± 0.033 b | 0.75 | 0.040–0.95 | 1.5 |
| Parameter | SL-v.1 | SL-v.2 | SL-v.3 | ||||
|---|---|---|---|---|---|---|---|
| +N | –N | +N | –N | +N | –N | ||
| Diameter, µm | Root | 1 290.8 ± 7.7 b | 280.6 ± 13.5 b | 289.0 ± 11.1 b | 276.7 ± 13.9 b | 414.4 ± 15.9 a | 370.0 ± 18.1 a |
| Stele | 68.11 ± 2.17 b | 65.56 ± 3.48 b | 73.29 ± 3.57 b | 65.44 ± 5.06 b | 105.64 ± 6.22 a | 98.72 ± 6.96 a | |
| The stele proportion in the root cross-sectional area, % | 5.78 ± 0.33 a | 5.75 ± 0.37 a | 6.57 ± 0.31 a | 5.75 ± 0.54 a | 6.59 ± 0.43 a | 7.20 ± 0.56 a | |
| Thickness of cortex parenchyma, µm | 61.85 ± 2.67 b | 57.71 ± 3.88 b | 57.55 ± 2.40 b | 58.54 ± 2.78 b | 94.71 ± 4.29 a | 87.86 ± 4.62 a | |
| Number of layers of cortex parenchyma, pcs. | 2.51 ± 0.09 a | 2.37 ± 0.11 ab | 2.03 ± 0.05 b | 2.05 ± 0.11 b | 2.69 ± 0.09 a | 2.30 ± 0.10 ab | |
| Thickness of cortex, µm | 111.32 ± 3.41 b | 107.52 ± 5.54 b | 107.83 ± 4.17 b | 105.63 ± 6.19 b | 154.39 ± 5.66 a | 135.64 ± 6.41 a | |
| Proportion of roots with aerenchyma, % | 78.05 | 55.56 | 71.05 | 63.64 | 85.71 | 81.48 | |
| Proportion of roots with thickened exodermis, % | 80.49 | 77.78 | 94.74 | 72.72 | 100.00 | 96.30 | |
| Proportion of roots with thickened endodermis, % | 0.00 | 0.00 | 7.89 | 0.00 | 14.29 | 7.41 | |
| Two-way ANOVA (Multivariate Tests of Significance) | Factor Varieties [V] | Factor Nitrogen [N] | [V] × [N] | ||||
| F = 9.285 | p < 0.001 | F = 0.968 | p = 0.463 | F = 1.511 | p = 0.093 | ||
| Tomato Variety | Treatment | Ntot., mg g−1 DW | Ntot., mg Plant−1 | ||
|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | ||
| SL-v.1 | +N | 1 8.990 ± 0.203 b | 18.267 ± 0.159 a | 4.746 ± 0.442 bc | 4.182 ± 0.276 b |
| –N | 6.985 ± 0.317 c | 8.074 ± 0.078 d | 0.257 ± 0.011 d | 0.129 ± 0.0373 c | |
| SL-v.2 | +N | 9.959 ± 0.320 b | 16.469 ± 0.296 b | 16.551 ± 2.852 a | 19.771 ± 2.558 a |
| –N | 7.725 ± 0.317 bc | 8.158 ± 0.101 d | 0.918 ± 0.075 cd | 0.478 ± 0.019 c | |
| SL-v.3 | +N | 11.756 ± 0.134 a | 13.330 ± 0.035 c | 8.139 ± 0.212 b | 2.091 ± 0.250 b |
| –N | 9.255 ± 0.309 b | 9.570 ± 0.370 d | 0.780 ± 0.033 cd | 0.227 ± 0.003 c | |
| Tomato Variety | Treatment | Ntot., mg 100 g−1 of ADS | Nalk.h., mg 100 g−1 of ADS | N–NO3−, mg 100 g−1 of ADS | N–NH4+, mg 100 g−1 of ADS | pHH2O |
|---|---|---|---|---|---|---|
| SL-v.1 | +N | 1 183.82 ± 3.10 a | 9.197 ± 0.381 bc | 0.608 ± 0.024 b | 0.042 ± 0.001 a | 6.49 ± 0.04 bc |
| –N | 163.83 ± 0.65 bc | 8.078 ± 0.292 c | 0.588 ± 0.005 b | 0.037 ± 0.002 b | 6.70 ± 0.03 a | |
| SL-v.2 | +N | 181.14 ± 3.12 a | 9.755 ± 0.558 bc | 0.689 ± 0.010 b | 0.018 ± 0.002 c | 6.58 ± 0.03 ab |
| –N | 184.56 ± 1.03 a | 9.540 ± 0.388 bc | 0.690 ± 0.019 b | 0.023 ± 0.001 c | 6.67 ± 0.01 a | |
| SL-v.3 | +N | 184.91 ± 1.93 a | 12.375 ± 0.302 a | 0.720 ± 0.022 b | 0.020 ± 0.001 c | 6.39 ± 0.04 c |
| –N | 174.63 ± 0.26 ab | 11.201 ± 0.381 ab | 0.867 ± 0.028 b | 0.026 ± 0.001 c | 6.73 ± 0.00 a | |
| Bulk soil | +N | 181.63 ± 3.54 a | 8.900 ± 0.413 c | 13.639 ± 0.350 a | 0.045 ± 0.002 a | 6.40 ± 0.02 c |
| –N | 158.00 ± 2.48 c | 8.725 ± 0.185 c | 0.901 ± 0.028 b | 0.033 ± 0.001 b | 6.73 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maleva, M.; Borisova, G.; Tugbaeva, A.; Malakheeva, A.; Voropaeva, O.; Ermoshin, A.; Betekhtina, A. Rhizobacteriome Diversity and Morphophysiological Features of Three Tomato Plant Varieties Under Nitrogen Deficiency. Nitrogen 2025, 6, 102. https://doi.org/10.3390/nitrogen6040102
Maleva M, Borisova G, Tugbaeva A, Malakheeva A, Voropaeva O, Ermoshin A, Betekhtina A. Rhizobacteriome Diversity and Morphophysiological Features of Three Tomato Plant Varieties Under Nitrogen Deficiency. Nitrogen. 2025; 6(4):102. https://doi.org/10.3390/nitrogen6040102
Chicago/Turabian StyleMaleva, Maria, Galina Borisova, Anastasia Tugbaeva, Alina Malakheeva, Olga Voropaeva, Alexander Ermoshin, and Anna Betekhtina. 2025. "Rhizobacteriome Diversity and Morphophysiological Features of Three Tomato Plant Varieties Under Nitrogen Deficiency" Nitrogen 6, no. 4: 102. https://doi.org/10.3390/nitrogen6040102
APA StyleMaleva, M., Borisova, G., Tugbaeva, A., Malakheeva, A., Voropaeva, O., Ermoshin, A., & Betekhtina, A. (2025). Rhizobacteriome Diversity and Morphophysiological Features of Three Tomato Plant Varieties Under Nitrogen Deficiency. Nitrogen, 6(4), 102. https://doi.org/10.3390/nitrogen6040102









