Microbial Alliance of Paenibacillus sp. SPR11 and Bradyrhizobium yuanmingense PR3 Enhances Nitrogen Fixation, Yield, and Salinity Tolerance in Black Gram Under Saline, Nutrient-Depleted Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Antagonistic Activity Against Plant Pathogens
2.2. Seed Germination Assay and Net House Evaluation of Growth and Nitrogenase Activity in Black Gram
2.2.1. Preparation of Inoculum
2.2.2. Seed Surface Sterilization and Inoculation
2.2.3. Seed Germination Assay
2.2.4. Measurement of Nitrogenase Activity and Growth Parameters in Black Gram
2.3. Field Site Description and Soil Analysis
2.4. Experimental Setup for Field Trials
2.5. Estimation of Physiological Parameters
2.5.1. Chlorophyll Content
2.5.2. Carotenoid Content
2.5.3. Electrolyte Leakage (EL)
- EC1 = Conductivity before incubation;
- EC2 = Conductivity after boiling water incubation.
2.5.4. Proline Content
2.6. Antioxidant Enzyme Activities
2.6.1. DPPH Radical Scavenging Activity
2.6.2. Catalase Activity (CAT)
2.6.3. Superoxide Dismutase Activity (SOD)
2.6.4. Estimation of Phenylalanine Ammonia Lyase (PAL)
2.6.5. Polyphenol Oxidase Activity Assay (PPO) Activity Assay
2.6.6. Estimation of Peroxidase (POD) Activity
2.7. Collection and Evaluation of Yield Parameters
2.8. Analysis of Plant Nutrient Uptake and Grain Nitrogen Content in Black Gram
2.9. Statistical Analysis
3. Results and Discussion
3.1. Antagonistic Activity of Paenibacillus sp. SPR11 Against Plant Pathogens
| Isolates | Inhibition in Dual Plate Assay (%) | |
| F. oxysporum | M. phaseolina | |
| Paenibacillus sp. SPR11 | 76% | 62% |
3.2. Effect of Co-Inoculation on Germination, Nodulation, and Nitrogen Fixation in Blackgram Under Salt Stress (Net House Study)
3.3. Soil Analysis
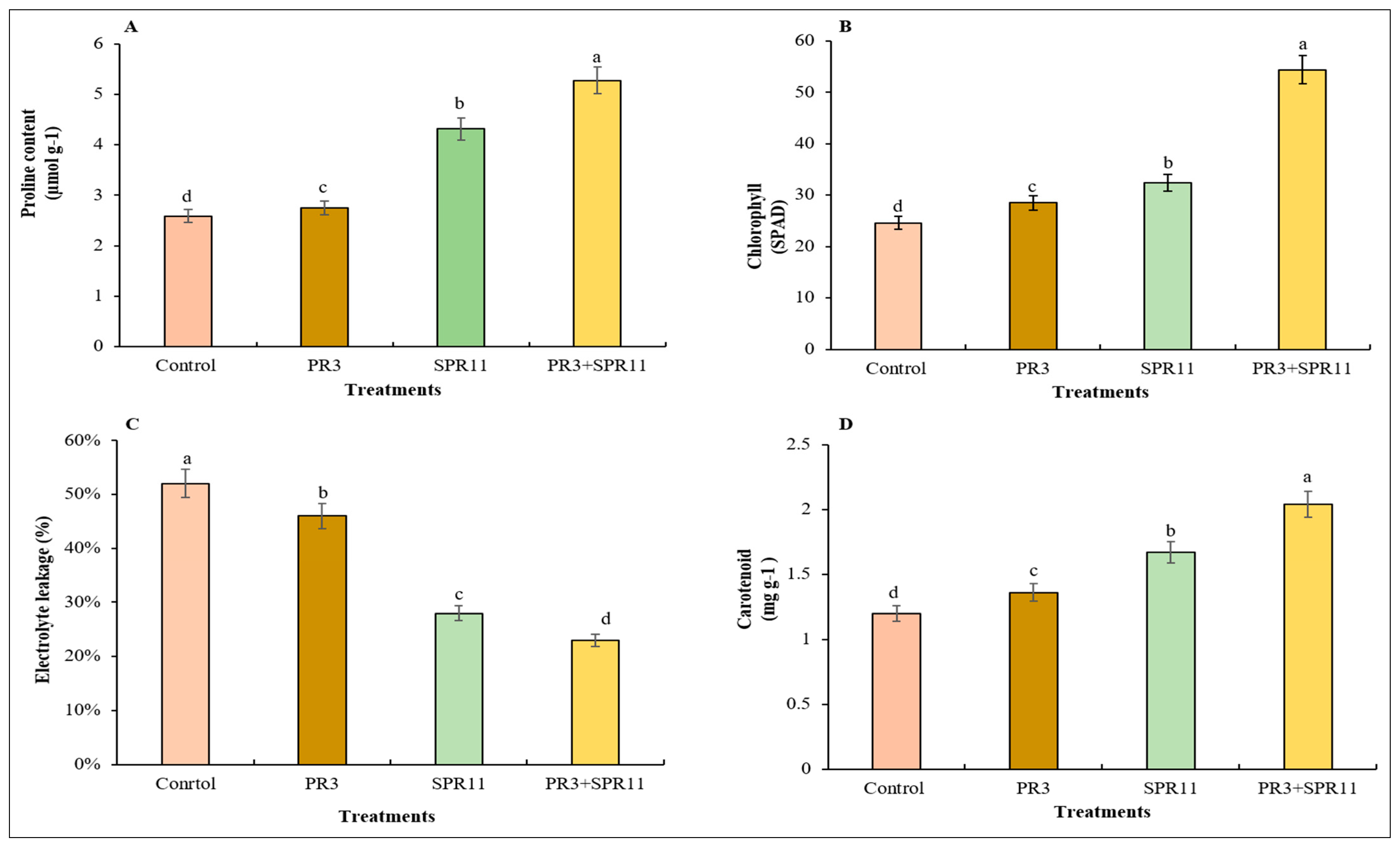
3.4. Effect of Co-Inoculation on Biochemical Parameters in Blackgram Under Saline Stress
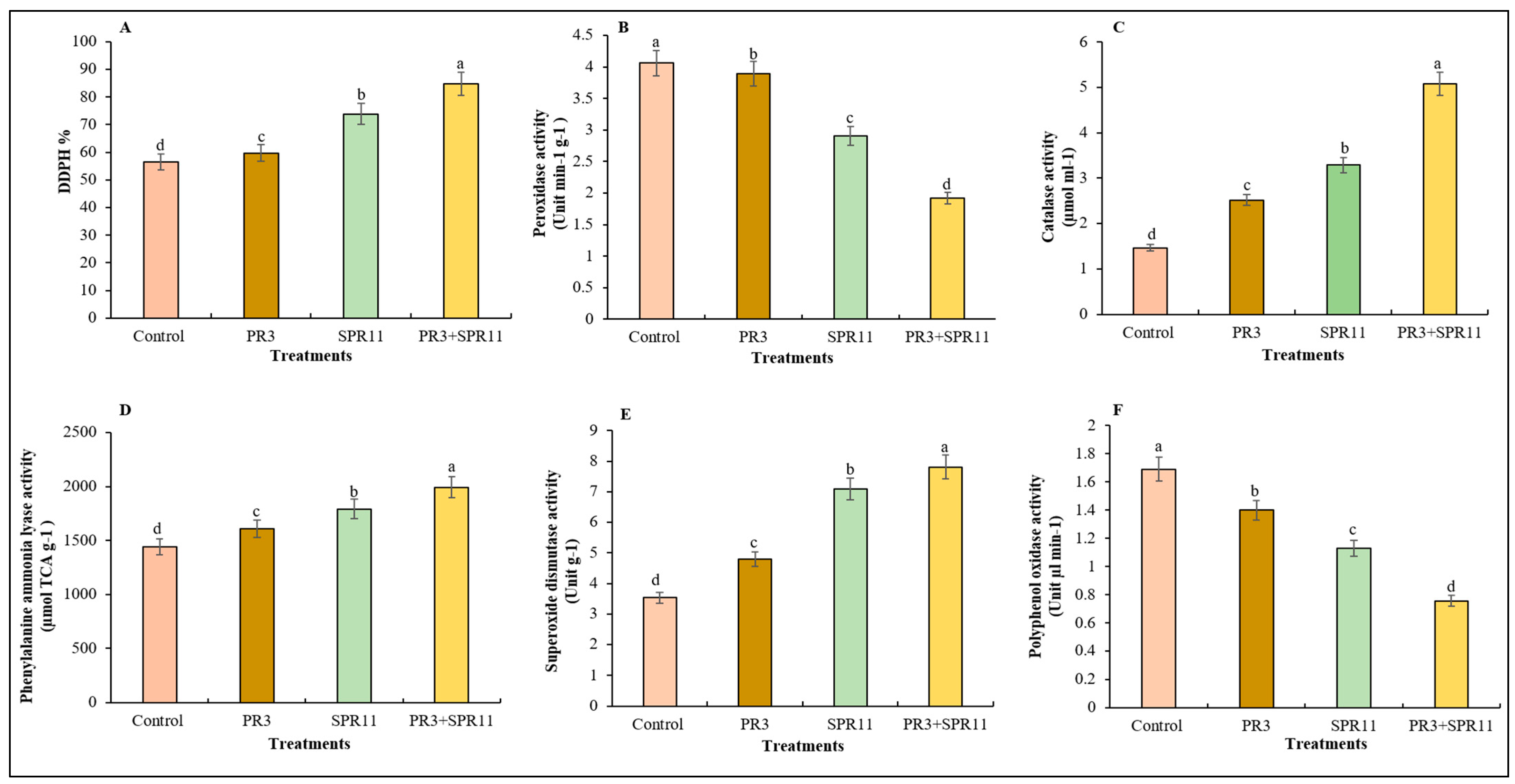
3.5. Effect of Co-Inoculation on Antioxidant Activities in Black Gram Under Field Conditions
3.6. Collection and Analysis of Yield Parameters
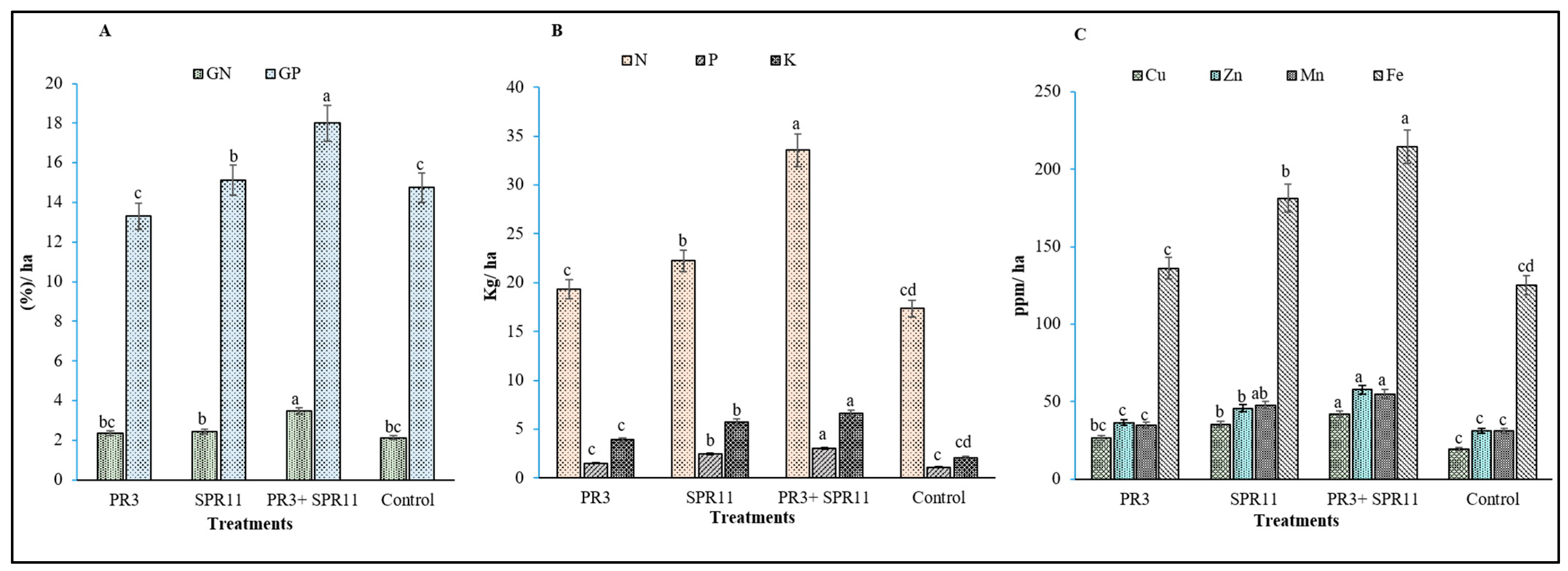
3.7. Analysis of Plant Nutrient Uptake and Grain Nitrogen Content in Black Gram
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, A.; Kumar, A.; Biswas, G. Exponential Population Growth and Global Food Security: Challenges and Alternatives. In Bioremediation of Emerging Contaminants from Soils; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–20. [Google Scholar]
- Mishra, P.; Mishra, J.; Arora, N.K. Plant Growth Promoting Bacteria for Combating Salinity Stress in Plants—Recent Developments and Prospects: A Review. Microbiol. Res. 2021, 252, 126861. [Google Scholar] [CrossRef]
- Chawla, S.; Jain, S.; Jain, V. Salinity Induced Oxidative Stress and Antioxidant System in Salt-Tolerant and Salt-Sensitive Cultivars of Rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2013, 22, 27–34. [Google Scholar] [CrossRef]
- Nagaraju, Y.; Mahadevaswamy; Gundappagol; Naik, N.M. Response of Black Gram to Seed Biopriming with Facultative Halophilic Bacteria under Salinity. J. Crop Weed 2021, 17, 226–234. [Google Scholar] [CrossRef]
- Indra, N.; Kauvyashree, A.S.; Swetha, D.; Asmina, M. Fungitoxic Effect of Inorganic Salts for the Management of Seed Borne Macrophomina phaseolina and Fusarium sp. Causing Charcoal Rot and Wilt Disease in Black Gram. Indian J. Agric. Res. 2019, 53, 208–212. [Google Scholar] [CrossRef]
- Kirova, E.; Kocheva, K. Physiological Effects of Salinity on Nitrogen Fixation in Legumes—A Review. J. Plant Nutr. 2021, 44, 2653–2662. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Sun, W.; Zhang, Q.; Diao, M.; Sun, J. Extreme Soil Salinity Reduces N and P Metabolism and Related Microbial Network Complexity and Community Immigration Rate. Environ. Res. 2025, 264, 120361. [Google Scholar] [CrossRef] [PubMed]
- Racioppo, A.; d’Amelio, A.; De Santis, A.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Potential Use of Plant Growth-Promoting Bacteria to Enhance Growth and Soil Fertility in Marginal Areas: Focus on the Apulia Region, Italy. Agronomy 2023, 13, 2983. [Google Scholar] [CrossRef]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of Plant Growth-Promoting Rhizobacteria (PGPR) on Plant Nutrition and Root Characteristics: Current Perspective. Plant Stress 2024, 11, 100341. [Google Scholar] [CrossRef]
- Tarolli, P.; Luo, J.; Park, E.; Barcaccia, G.; Masin, R. Soil Salinization in Agriculture: Mitigation and Adaptation Strategies Combining Nature-Based Solutions and Bioengineering. iScience 2024, 27, 102420. [Google Scholar] [CrossRef]
- Etesami, H. Root Nodules of Legumes: A Suitable Ecological Niche for Isolating Non-Rhizobial Bacteria with Biotechnological Potential in Agriculture. Curr. Res. Biotechnol. 2022, 4, 78–86. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Li, Y.; Guan, G.; Chen, S. Paenibacillus Strains with Nitrogen Fixation and Multiple Beneficial Properties for Promoting Plant Growth. PeerJ 2019, 7, e7445. [Google Scholar] [CrossRef]
- Swarnalakshmi, K.; Yadav, V.; Tyagi, D.; Dhar, D.W.; Kannepalli, A.; Kumar, S. Significance of Plant Growth Promoting Rhizobacteria in Grain Legumes: Growth Promotion and Crop Production. Plants 2020, 9, 1596. [Google Scholar] [CrossRef]
- Sukweenadhi, J.; Balusamy, S.R.; Kim, Y.J.; Lee, C.H.; Kim, Y.J.; Koh, S.C.; Yang, D.C. A Growth-Promoting Bacterium, Paenibacillus yonginensis DCY84^T, Enhanced Salt Stress Tolerance by Activating Defense-Related Systems in Panax ginseng. Front. Plant Sci. 2018, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Ye, X.P.; Hu, Y.Y.; Tang, Z.X.; Zhang, T.; Zhou, H.; Zhou, T.; Bai, X.L.; Pi, E.X.; Xie, B.H.; et al. Exopolysaccharides of Paenibacillus polymyxa: A Review. Int. J. Biol. Macromol. 2024, 261, 129663. [Google Scholar] [CrossRef]
- Costa, A.; Corallo, B.; Amarelle, V.; Stewart, S.; Pan, D.; Tiscornia, S.; Fabiano, E. Paenibacillus sp. Strain UY79, Isolated from a Root Nodule of Arachis villosa, Displays a Broad Spectrum of Antifungal Activity. Appl. Environ. Microbiol. 2022, 88, e01645-21. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.K.; Srivastava, A.K.; Singh, R.; Srivastava, A.K. Stress-Relieving Plant Growth-Promoting Bacterial Co-Inoculation Enhances Nodulation and Nitrogen Uptake in Black Gram under Nitrogen-Free Saline Conditions. Front. Microbiol. 2025, 15, 1516748. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Vemula, A.; Samineni, S.; Rathore, A. Influence of Diazotrophic Bacteria on Nodulation, Nitrogen Fixation, Growth Promotion and Yield Traits in Five Cultivars of Chickpea. Biocatal. Agric. Biotechnol. 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and Soil Acidity. In Methods of Soil Analysis: Part 3—Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 475–489. [Google Scholar]
- Rhoades, J.D.; Chanduvi, F.; Lesch, S.M. Soil Salinity Assessment: Methods and Interpretation of Electrical Conductivity Measurements; FAO: Rome, Italy, 1999; No. 57. [Google Scholar]
- Walkley, A.J.; Black, I.A. Estimation of Soil Organic Carbon by the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A Rapid Procedure for the Estimation of Available Nitrogen in Soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. USDA Circ. 1954, 939, 1–19. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Lindsay, W.L.; Martens, D.C. Testing Soils for Copper, Iron, Manganese, and Zinc. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 229–264. [Google Scholar]
- Zhang, R.; Yang, P.; Liu, S.; Wang, C.; Liu, J. Evaluation of the Methods for Estimating Leaf Chlorophyll Content with SPAD Chlorophyll Meters. Remote Sens. 2022, 14, 5144. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Bastam, N.; Baninasab, B.; Ghobadi, C. Improving Salt Tolerance by Exogenous Application of Salicylic Acid in Seedlings of Pistachio. Plant Growth Regul. 2013, 69, 275–284. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.A.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Laoutari, S.; Litskas, V.D.; Stavrinides, M.C.; Tzortzakis, N. Effects of Water Stress on Lavender and Sage Biomass Production, Essential Oil Composition and Biocidal Properties against Tetranychus urticae (Koch). Sci. Hortic. 2016, 213, 96–103. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Up-Regulation of Antioxidant and Glyoxalase Systems by Exogenous Glycinebetaine and Proline in Mung Bean Confer Tolerance to Cadmium Stress. Physiol. Mol. Biol. Plants 2010, 16, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-Tolerant Plant Growth-Promoting Bacillus pumilus Strain JPVS11 to Enhance Plant Growth Attributes of Rice and Improve Soil Health under Salinity Stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Brueske, C.H. Phenylalanine ammonia lyase activity in tomato roots infected and resistant to the root-knot nematode, Meloidogyne incognita. Physiol. Plant Pathol. 1980, 16, 409–414. [Google Scholar] [CrossRef]
- Jockusch, H. The Role of Host Genes, Temperature and Polyphenoloxidase in the Necrotization of TMV-Infected Tobacco Tissue. Phytopathology 1966, 56, 789–794. [Google Scholar] [CrossRef]
- Kumari, S.; Vaishnav, A.; Jain, S.; Varma, A.; Choudhary, D.K. Bacterial-Mediated Induction of Systemic Tolerance to Salinity with Expression of Stress Alleviating Enzymes in Soybean (Glycine max L. Merrill). J. Plant Growth Regul. 2015, 34, 558–573. [Google Scholar] [CrossRef]
- Yadav, S.K.; Yogeshwar, S.; Yadav, M.K.; Subhash, B.; Kalyan, S. Effect of Organic Nitrogen Sources on Yield, Nutrient Uptake and Soil Health under Rice (Oryza sativa)-Based Cropping Sequence. Indian J. Agric. Sci. 2013, 83, 170–175. [Google Scholar]
- Dobrzyński, J.; Naziębło, A. Paenibacillus as a Biocontrol Agent for Fungal Phytopathogens: Is P. polymyxa the Only One worth Attention? Microb. Ecol. 2024, 87, 134. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Biswas, A.K.; De, B. Influence of Sodium Chloride on Growth and Metabolic Reprogramming in Non-Primed and Halo-Primed Seedlings of Black Gram (Vigna mungo L.). Protoplasma 2020, 257, 1559–1583. [Google Scholar] [CrossRef]
- Vennila, S.; Elangaimannan, R.; Mathiazhagan, P.; LubnaArshiya, S. Salt Stress Responses in Early Seedling Growth Characteristics of Black Gram [Vigna mungo (L.) Hepper] Genotypes. Int. J. Plant Soil Sci. 2023, 35, 1021–1037. [Google Scholar] [CrossRef]
- Win, K.T.; Wasai-Hara, S.; Tanaka, F.; Oo, A.Z.; Minamisawa, K.; Shimoda, Y.; Imaizumi-Anraku, H. Synergistic N2-Fixation and Salt Stress Mitigation in Soybean through Dual Inoculation of ACC Deaminase-Producing Pseudomonas and Bradyrhizobium. Sci. Rep. 2023, 13, 17050. [Google Scholar] [CrossRef]
- Patel, A.; Verma, S.; Singh, S.K.; Singh, R.K. Soil Fertility Status of Jaunpur District in Eastern Uttar Pradesh. J. Pharmacogn. Phytochem. 2017, 6, 949–952. [Google Scholar]
- Mahdavian, K. Application of Salicylic Acid on Chlorophyll, Carotenoids, and Proline in Radish under Salinity Stress. Proc. Natl. Acad. Sci. India B Biol. Sci. 2023, 93, 809–818. [Google Scholar] [CrossRef]
- Singh, A.; Prasad, V. Mitigation of Salinity-Induced Negative Impacts by Salt-Tolerant Plant Growth-Promoting Rhizobacteria Bacillus flexus in Mustard (Brassica juncea L.). Front. Microbiol. 2025, 16, 1638366. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) Augments Salt Tolerance in Soybean (Glycine max L.) by Modulating Root System Architecture, Antioxidant Defense Systems and Stress-Responsive Genes Expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef]
- Yilmaz, H.; Kulaz, H. The Effects of Plant Growth-Promoting Rhizobacteria on Antioxidant Activity in Chickpea (Cicer arietinum L.) under Salt Stress. Legum. Res. 2019, 42, 72–76. [Google Scholar] [CrossRef]
- Hassan, A.H.; Alkhalifah, D.H.M.; Al Yousef, S.A.; Beemster, G.T.; Mousa, A.S.; Hozzein, W.N.; AbdElgawad, H. Salinity Stress Enhances the Antioxidant Capacity of Bacillus and Planococcus Species Isolated from Saline Lake Environment. Front. Microbiol. 2020, 11, 561816. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Moh, S.M.; Moe, K.; Yamakawa, T. Effects of Co-Inoculation of Bradyrhizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 on Plant Growth, Nodulation, Nitrogen Fixation, Nutrient Uptake, and Yield of Soybean in Field Conditions. Soil Sci. Plant Nutr. 2018, 64, 222–229. [Google Scholar] [CrossRef]
- Korir, H.; Mungai, N.W.; Thuita, M.; Hamba, Y.; Masso, C. Co-Inoculation Effect of Rhizobia and Plant Growth-Promoting Rhizobacteria on Common Bean Growth in Low-Phosphorus Soil. Front. Plant Sci. 2017, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Abdiev, A.; Khaitov, B.; Toderich, K.; Park, K.W. Growth, Nutrient Uptake, and Yield Parameters of Chickpea (Cicer arietinum L.) Enhanced by Rhizobium and Azotobacter Inoculations in Saline Soil. J. Plant Nutr. 2019, 42, 2703–2714. [Google Scholar] [CrossRef]
- da Silva, F.B.; Barbosa, J.Z.; Tiecher, T.; Borin, J.B.M.; Treichel, B.; de Sá, E.L.S. Species-Dependent Effect of Rhizobacteria Co-Inoculation in Legume Plants: A Global Meta-Analysis. Rhizosphere 2024, 30, 100869. [Google Scholar] [CrossRef]
- Jabborova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-Inoculation of Rhizobacteria Promotes Growth, Yield, and Nutrient Contents in Soybean and Improves Soil Enzymes and Nutrients under Drought Conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sharma, S.; Sharma, S.; Kumar, V. Synergistic Effect of Bio-Inoculants on Yield, Nodulation, and Nutrient Uptake of Chickpea (Cicer arietinum L.) under Rainfed Conditions. J. Plant Nutr. 2019, 42, 374–383. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Nafady, N.A.; Bashandy, S.R.; Hassan, A.A. Mitigation of Salt-Stress Effects on Nodulation, Nitrogen Fixation, and Growth of Chickpea (Cicer arietinum L.) by Triple Microbial Inoculation. Rhizosphere 2019, 10, 100148. [Google Scholar] [CrossRef]


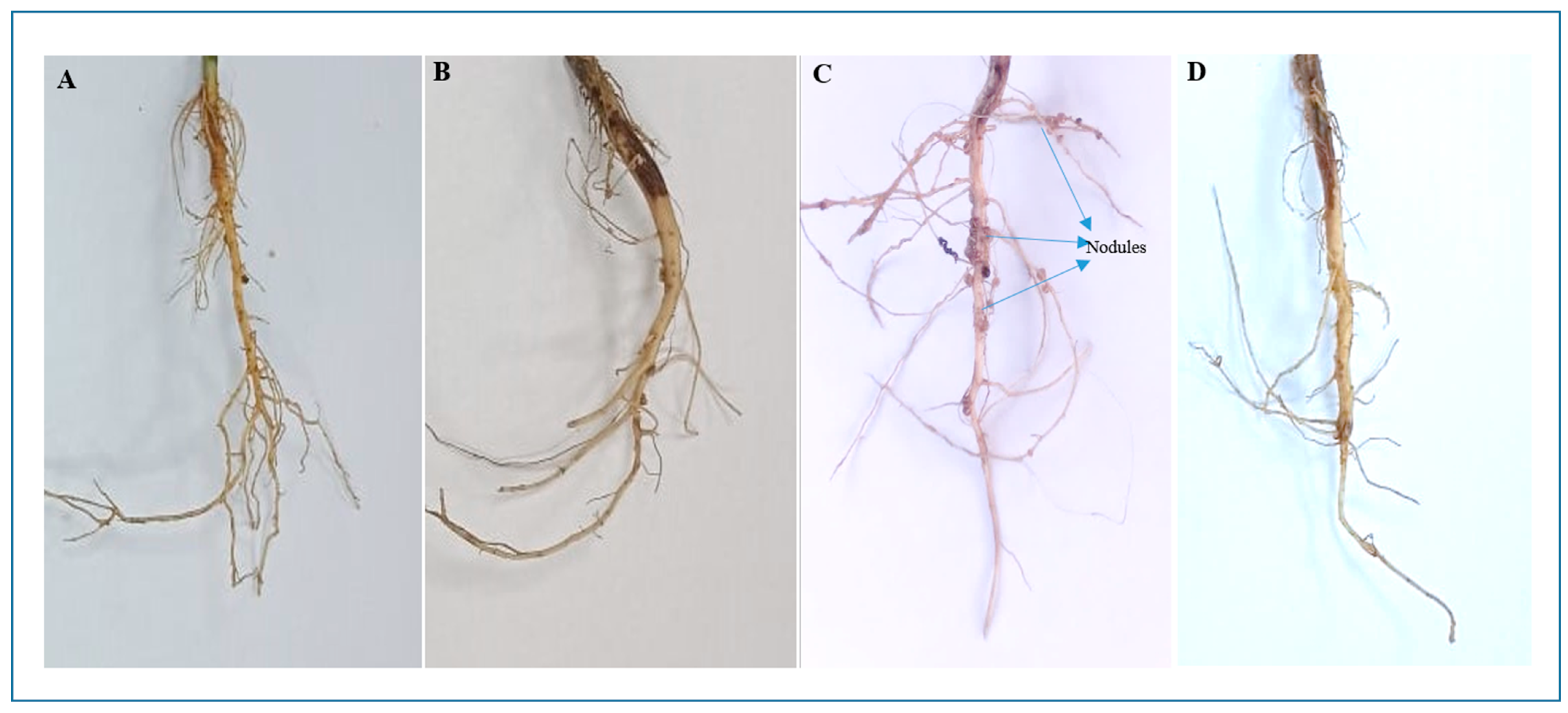
| Seed Germination Test | Net House Evaluation (300 mM NaCl) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Seed Germination (%) Non-Saline Saline | Seed Root Length (cm) Non-Saline Saline | RL (cm) | RFW (mg) | SL (cm) | SFW (mg) | NC (mg) | NFW (mg) | NDW (mg) | NF (μmol/mg FW h−1) | ||
| PR3 | 100% | 70% | 2.16 c | 0.86 c | 17.13 c | 105.40 c | 22.37 c | 248.30 c | 13.00 b | 106.37 b | 9.54 b | 3.96 b |
| SPR11 | 100% | 100% | 3.92 b | 2.75 b | 31.25 b | 312.23 b | 39.47 a | 417.57 b | 0.00 c | 0.000 c | 0.00 c | 0.00 c |
| PR3 + SPR11 | 100% | 100% | 5.95 a | 3.63 a | 39.30 a | 386.24 a | 41.37 a | 553.20 a | 61.56 a | 723.23 a | 66.87 a | 8.32 a |
| Control | 86% | 56.56% | 1.88 cd | 0.26 cd | 15.17 cd | 89.52 d | 19.57 d | 221.54 cd | 0.00 c | 0.00 c | 0.00 c | 0.00 c |
| Treatment | RL (cm) | RFW (mg) | SL (cm) | SFW (mg) | NC (mg) | NFW (mg) | NDW (mg) | PD (Plant−1) | SN (Plant−1) | SW (g Plant−1) | Grain yield (q ha1) | SY (q ha1) | Total biomass (q ha1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR3 | 24.68 bc | 124.93 c | 26.40 c | 293.44 c | 8.00 bc | 24.66 b | 7.18 b | 17.67 b | 82.23 c | 6.37 bc | 4.82 bc | 8.29 bc | 13.11 c |
| SPR11 | 27.33 a | 223.70 b | 38.34 b | 437.70 b | 9.32.00 b | 21.300 bc | 8.37 b | 18.12 b | 126.87 b | 7.26 b | 5.68 b | 9.72 b | 18.40 b |
| PR3 + SPR11 | 27.71 a | 284.00 a | 42.54 a | 469.66 a | 31.71 a | 158.70 a | 31.34 a | 32.54 a | 242.67 a | 11.38 a | 8.18 a | 15.55 a | 26.73 a |
| Control | 25.72 b | 119.60 cd | 23.00 d | 262.71 cd | 5.62.00 c | 15.23 c | 4.97 c | 12.31 c | 61.57 d | 5.86 bc | 3.91 c | 3.57 c | 11.48 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, P.K.; Srivastava, A.K.; Singh, R.; Srivastava, A.K. Microbial Alliance of Paenibacillus sp. SPR11 and Bradyrhizobium yuanmingense PR3 Enhances Nitrogen Fixation, Yield, and Salinity Tolerance in Black Gram Under Saline, Nutrient-Depleted Soils. Nitrogen 2025, 6, 66. https://doi.org/10.3390/nitrogen6030066
Tiwari PK, Srivastava AK, Singh R, Srivastava AK. Microbial Alliance of Paenibacillus sp. SPR11 and Bradyrhizobium yuanmingense PR3 Enhances Nitrogen Fixation, Yield, and Salinity Tolerance in Black Gram Under Saline, Nutrient-Depleted Soils. Nitrogen. 2025; 6(3):66. https://doi.org/10.3390/nitrogen6030066
Chicago/Turabian StyleTiwari, Praveen Kumar, Anchal Kumar Srivastava, Rachana Singh, and Alok Kumar Srivastava. 2025. "Microbial Alliance of Paenibacillus sp. SPR11 and Bradyrhizobium yuanmingense PR3 Enhances Nitrogen Fixation, Yield, and Salinity Tolerance in Black Gram Under Saline, Nutrient-Depleted Soils" Nitrogen 6, no. 3: 66. https://doi.org/10.3390/nitrogen6030066
APA StyleTiwari, P. K., Srivastava, A. K., Singh, R., & Srivastava, A. K. (2025). Microbial Alliance of Paenibacillus sp. SPR11 and Bradyrhizobium yuanmingense PR3 Enhances Nitrogen Fixation, Yield, and Salinity Tolerance in Black Gram Under Saline, Nutrient-Depleted Soils. Nitrogen, 6(3), 66. https://doi.org/10.3390/nitrogen6030066







