Effect of Biochar-Coated Urea on Soil Nitrogen, Plant Uptake, and Sweet Corn Yield in Sandy Soil
Abstract
1. Introduction
2. Materials and Methods
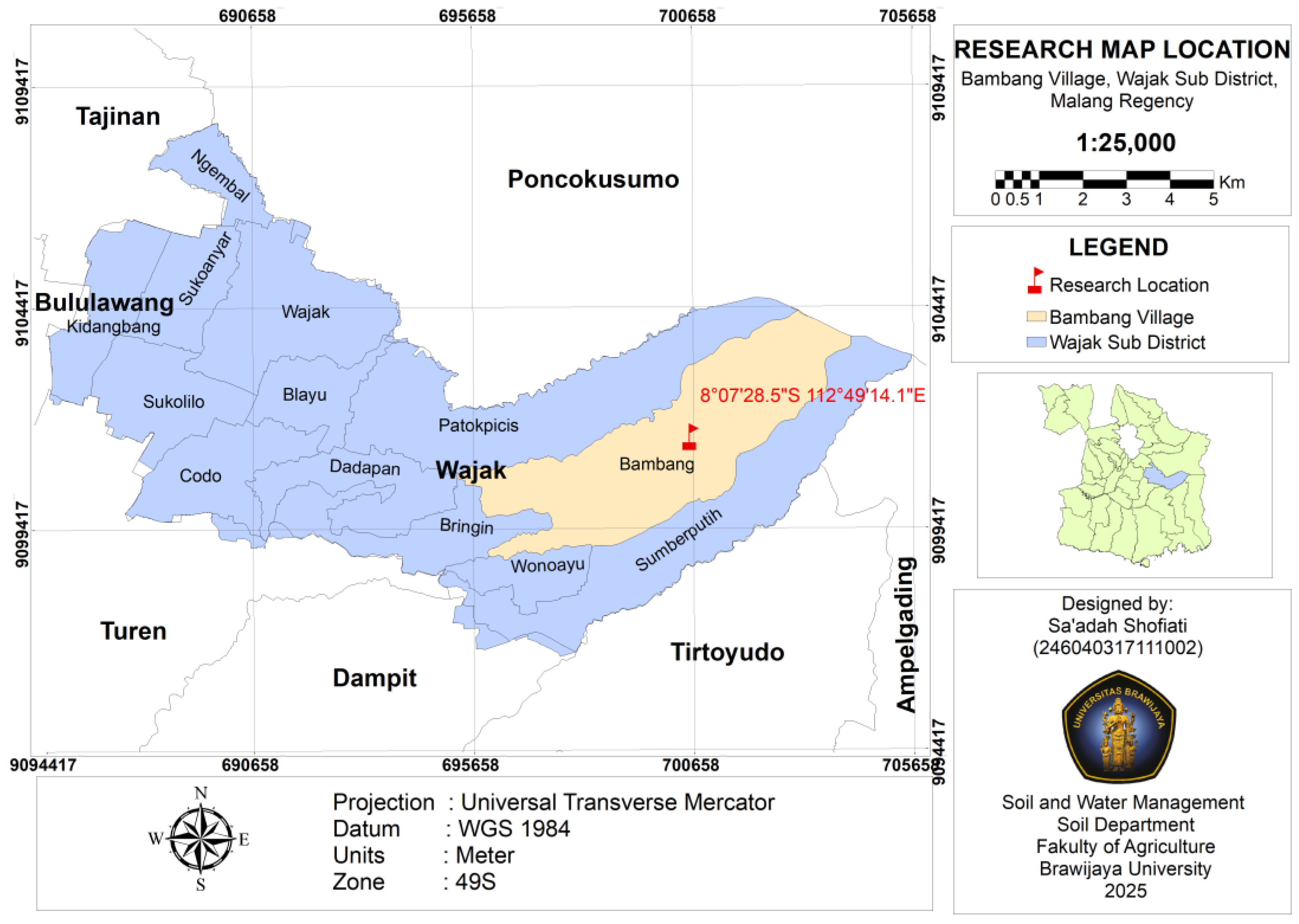
2.1. Study Site
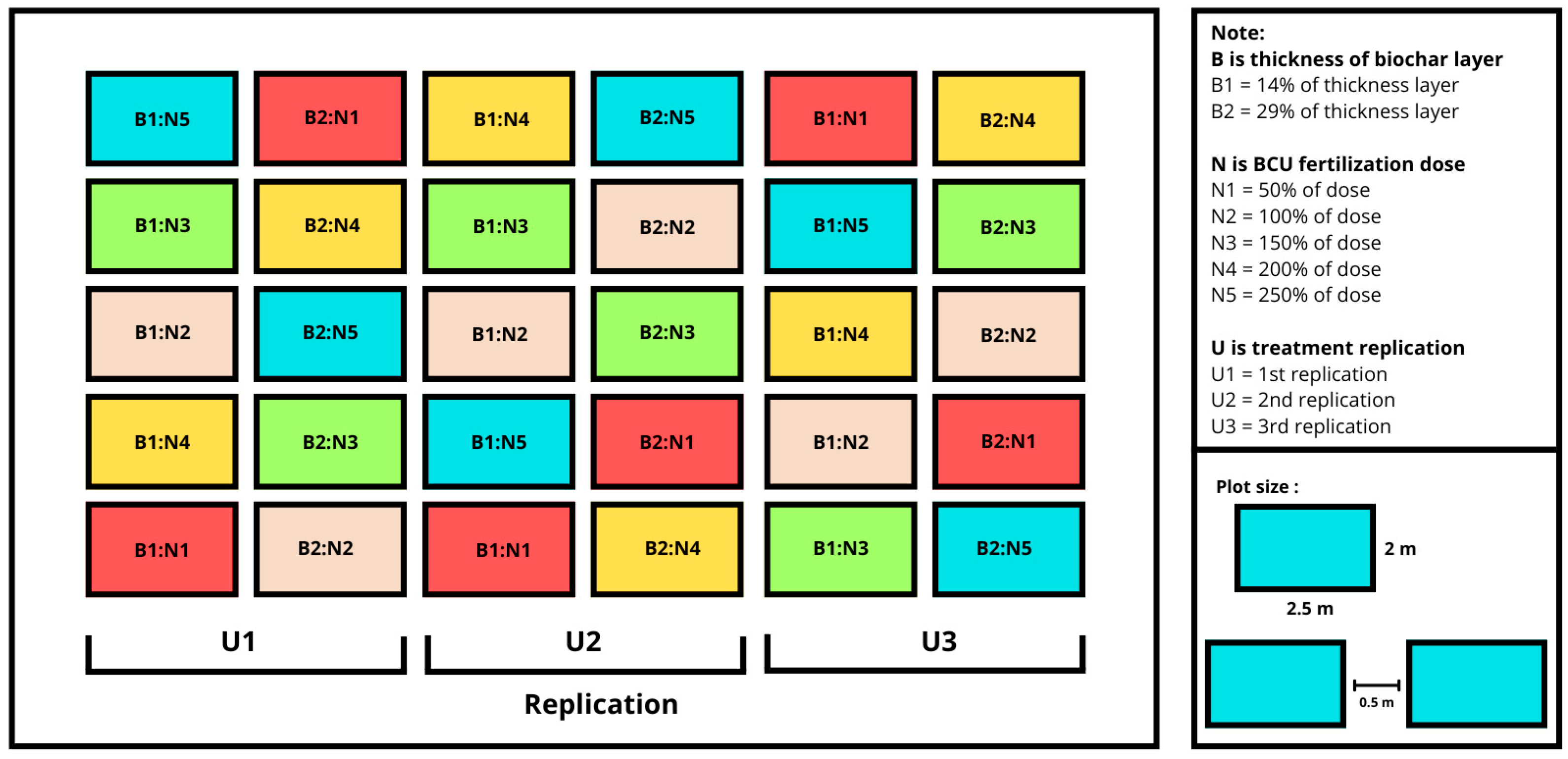
2.2. Experimental Design
2.3. Biochar Preparation
2.4. BCU Preparation
2.5. Field Experiment
2.6. Soil Sampling
2.7. Soil N Measurement
2.8. Plant N and N-Use Efficiency Measurement
2.9. Growth and Yield Measurement
2.10. Statistical Analysis
3. Results
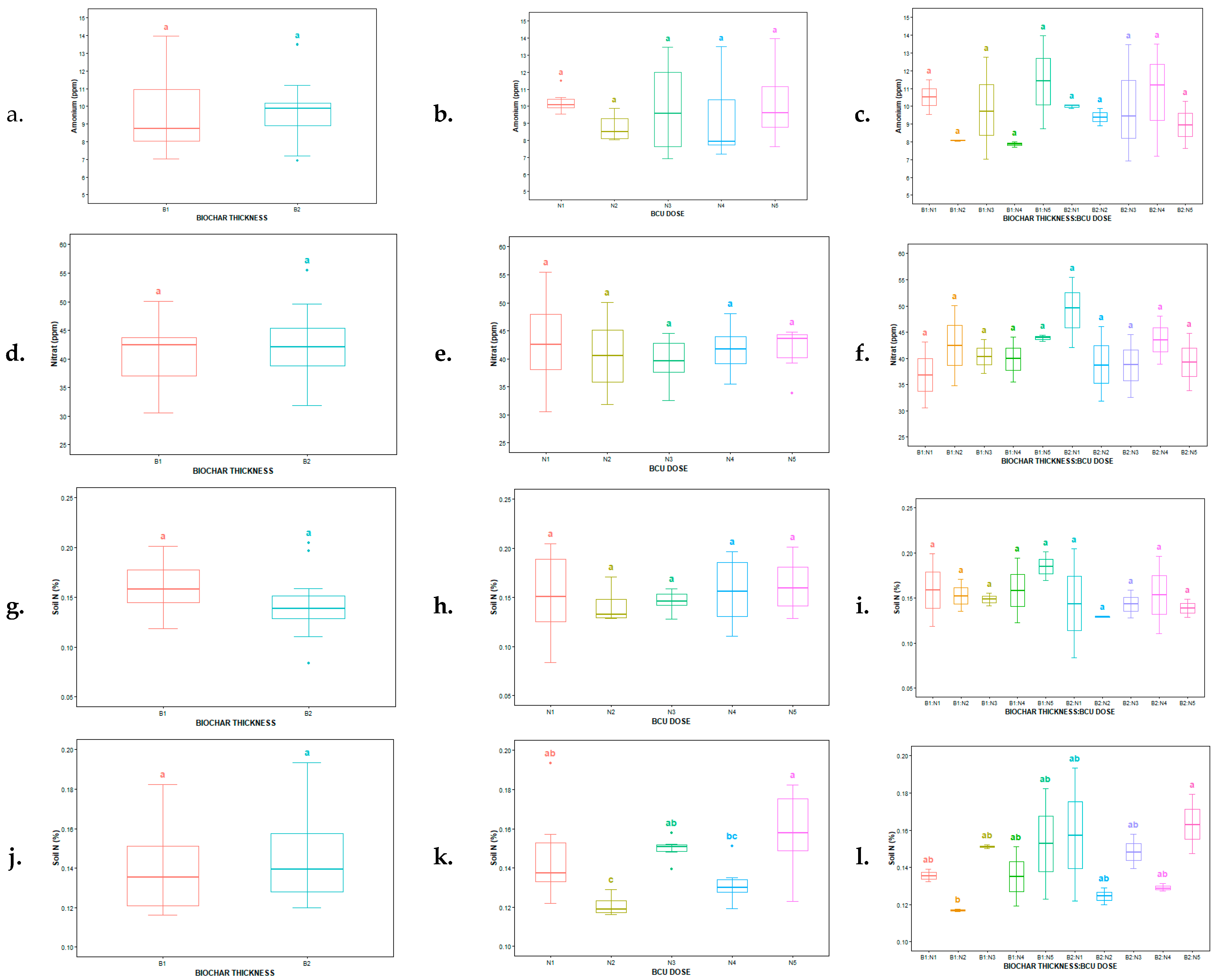
3.1. Effect of Biochar-Coated Urea on Total and Available Soil N
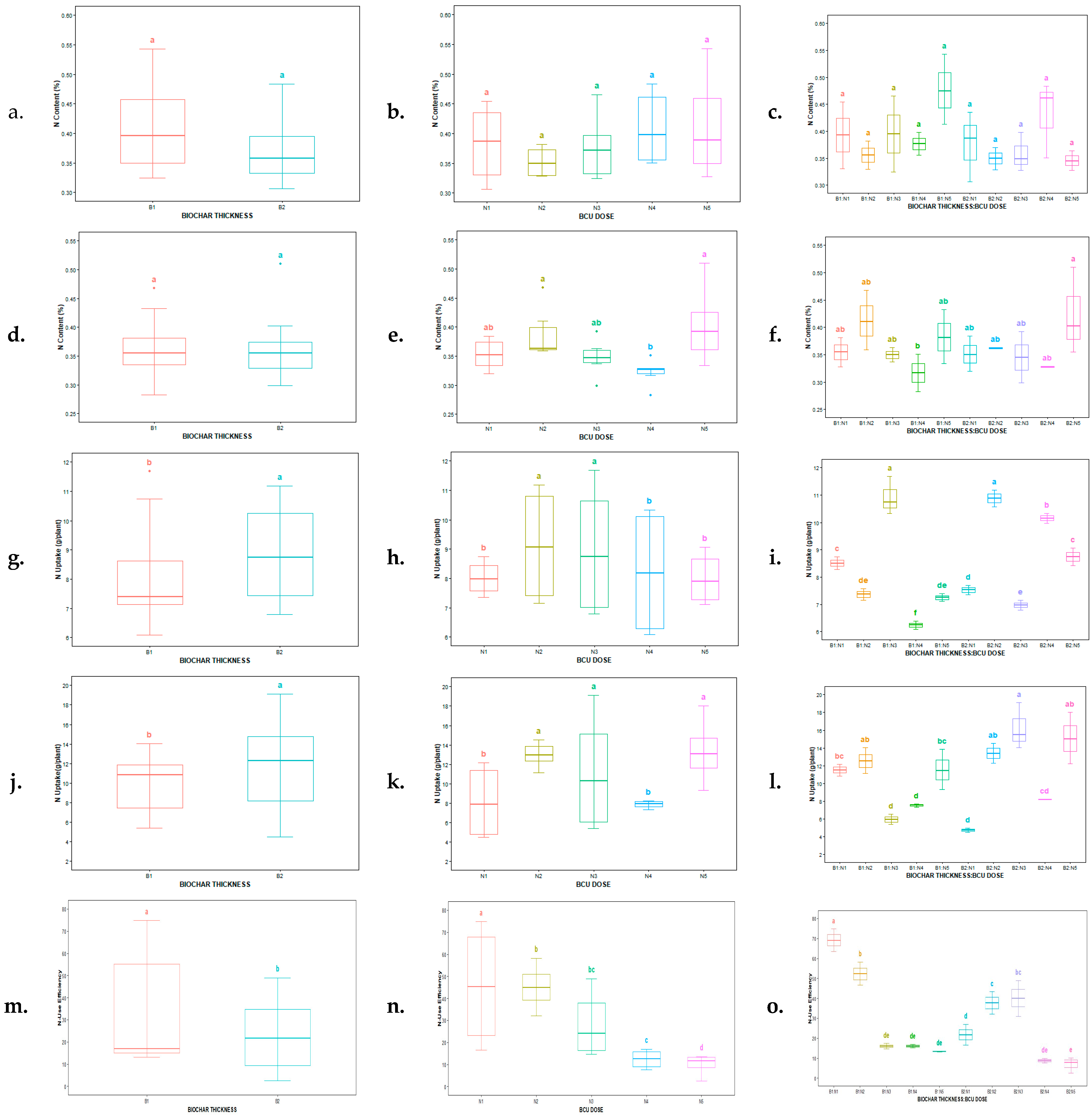
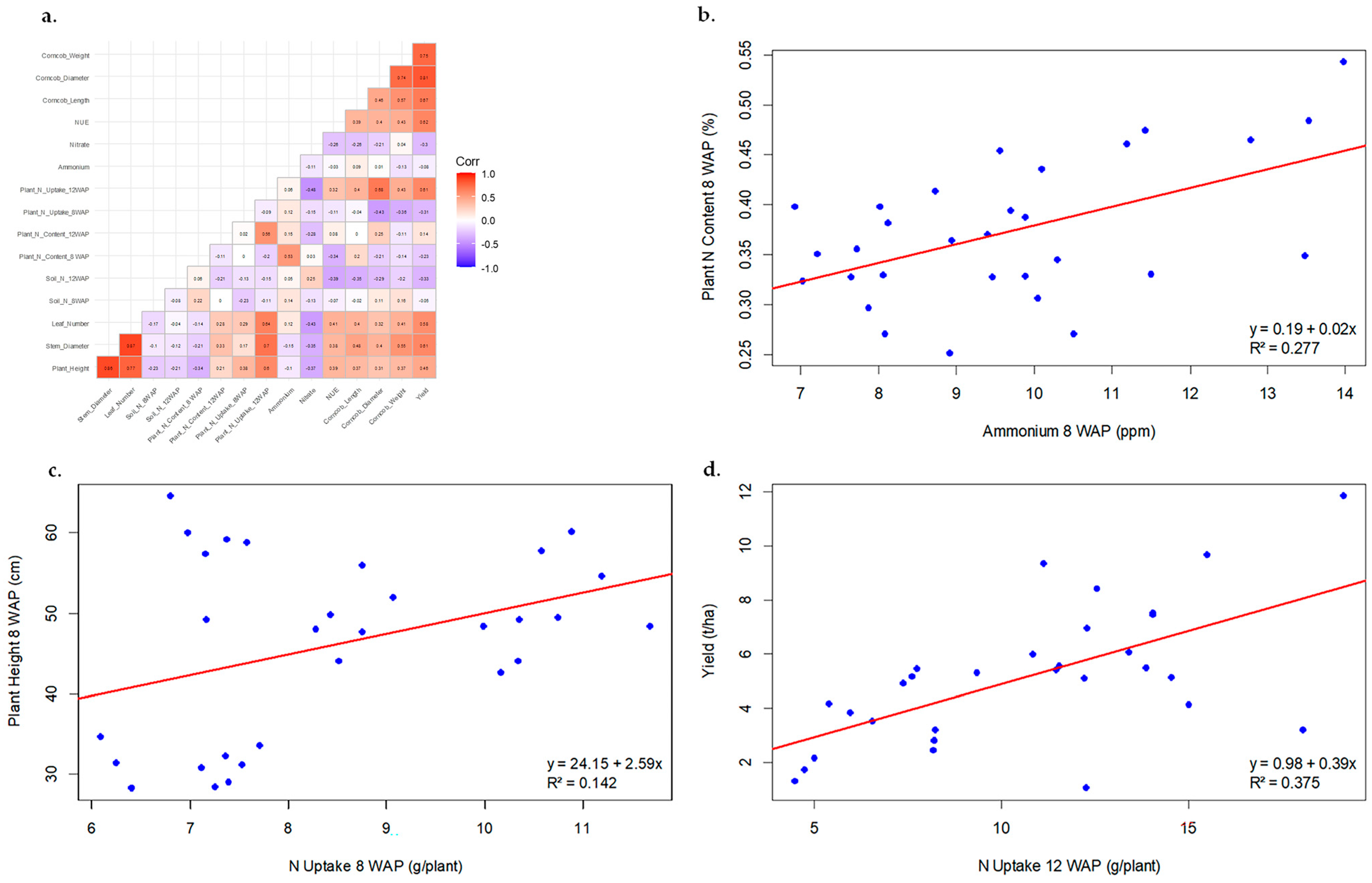
3.2. Effect of Biochar-Coated Urea on N Content, N Uptake, and N-Use Efficiency
3.3. Effect of Biochar-Coated Urea on Plant Growth
3.4. Effect of Biochar-Coated Urea on Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.G.K.S.; Vadivel, R.T.K.D.; Raj, R.; Pooniya, V.; et al. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Li, J.; Huang, D. The Utilization and Roles of Nitrogen in Plants. Forests 2024, 15, 1191. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Jalpa, L.; Mylavarapu, R.S.; Hochmuth, G.J.; Wright, A.L.; van Santen, E. Apparent recovery and efficiency of nitrogen fertilization in tomato grown on sandy soils. HortTechnology 2020, 30, 204–211. [Google Scholar] [CrossRef]
- Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem. Sustainability 2021, 13, 2400. [Google Scholar] [CrossRef]
- Monib, A.W.; Niazi, P.; Barai, S.M.; Sawicka, B.; Baseer, A.Q.; Nikpay, A.; Saleem Fahmawi, S.M.; Singh, D.; Alikhail, M.; Thea, B. Nitrogen Cycling Dynamics: Investigating Volatilization and its Interplay with N2 Fixation. J. Res. Appl. Sci. Biotechnol. 2024, 3, 17–31. [Google Scholar] [CrossRef]
- Wen, S.; Cui, N.; Wang, Y.; Gong, D.; Xing, L.; Wu, Z.; Zhang, Y.; Wang, Z. Determining Effect of Fertilization on Reactive Nitrogen Losses through Nitrate Leaching and Key Influencing Factors in Chinese Agricultural Systems. Agric. Water Manag. 2024, 303, 109055. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Dong, G.; Du, Z.; Wu, W.; Chadwick, D.; Bol, R. The Importance of Ammonia Volatilization in Estimating the Efficacy of Nitrification Inhibitors to Reduce N2O Emissions: A Global Meta-Analysis. Environ. Pollut. 2021, 271, 116365. [Google Scholar] [CrossRef]
- Bhatt, R.; Kunal; Moulick, D.; Bárek, V.; Brestic, M.; Gaber, A.; Skalicky, M.; Hossain, A. Sustainable strategies to limit nitrogen loss in agriculture through improving its use efficiency—Aiming to reduce environmental pollution. J. Agric. Food Res. 2025, 22, 101957. [Google Scholar] [CrossRef]
- Abdallah, A.M. The Effect of Hydrogel Particle Size on Water Retention Properties and Availability under Water Stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]
- Arunrat, N.; Kongsurakan, P.; Sereenonchai, S.; Hatano, R. Soil Organic Carbon in Sandy Paddy Fields of Northeast Thailand: A Review. Agronomy 2020, 10, 1061. [Google Scholar] [CrossRef]
- Siswanto; Mindari, W.; Santoso, S.B.; Rahayu, R.D. Effectiveness of Ameliorant Humic Acid and Silica Based on Availability of Sandy Soil Nitrogen for Rice. Nusant. Sci. Technol. Proc. 2022, 24, 340–343. [Google Scholar] [CrossRef]
- de Jesus Duarte, S.; Glaser, B.; Pellegrino Cerri, C.E. Effect of Biochar Particle Size on Physical, Hydrological and Chemical Properties of Loamy and Sandy Tropical Soils. Agronomy 2019, 9, 165. [Google Scholar] [CrossRef]
- Sandhu, V.; Lamba, J.; Kaur, P.; Malhotra, K.; Way, T.R.; Balkcom, K.S.; Prasad, R. Effect of Cover Crops on Phosphorus and Trace Metal Leaching in Agricultural Soils. Agric. Water Manag. 2025, 309, 109343. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, T. Soil Pore Structure and Its Research Methods: A Review. Soil Water Res. 2024, 19, 1–24. [Google Scholar] [CrossRef]
- Md Motasim, A.; Samsuri, A.W.; Nabayi, A.; Akter, A.; Haque, M.A.; Sukor, A.S.A.; Adibah, A.M. Urea application in soil: Processes, losses, and alternatives—A review. Discov. Agric. 2024, 2, 42. [Google Scholar] [CrossRef]
- Hussain, A.; Jahan, N.; Jabeen, Z.; Rehman, K.U.; Rafeeq, H.; Bilal, M.; Iqbal, H.M. Synergistic effect of urease and nitrification inhibitors in the reduction of ammonia volatilization. Water Air Soil Pollut. 2021, 232, 303. [Google Scholar] [CrossRef]
- Suwardi; Suryaningtyas, D.T.; Ghofar, A.; Rosjidi, M.; Mustafa, A.; Saputra, H. Effect of polyethylene glycol and humic acid coating on NPK release from controlled-release fertilizer. Sci. World J. 2024, 2024, 5510660. [Google Scholar] [CrossRef]
- Incrocci, L.; Maggini, R.; Cei, T.; Carmassi, G.; Botrini, L.; Filippi, F.; Clemens, R.; Terrones, C.; Pardossi, A. Innovative Controlled-Release Polyurethane-Coated Urea Could Reduce N Leaching in Tomato Crop in Comparison to Conventional and Stabilized Fertilizers. Agronomy 2020, 10, 1827. [Google Scholar] [CrossRef]
- Amalina, F.; Syukor Abd Razak, A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar production techniques utilizing biomass waste-derived materials and environmental applications—A review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Kumar, M.; Dutta, S.; You, S.; Luo, G.; Zhang, S.; Show, P.L.; Sawarkar, A.D.; Singh, L.; Tsang, D.C.W. A Critical Review on Biochar for Enhancing Biogas Production from Anaerobic Digestion of Food Waste and Sludge. J. Clean. Prod. 2021, 305, 127143. [Google Scholar] [CrossRef]
- Rambhatla, N.; Panicker, T.F.; Mishra, R.K.; Manjeshwar, S.K.; Sharma, A. Biomass Pyrolysis for Biochar Production: Study of Kinetics Parameters and Effect of Temperature on Biochar Yield and Its Physicochemical Properties. Results Eng. 2025, 25, 103679. [Google Scholar] [CrossRef]
- Khater, E.-S.; Bahnasawy, A.; Hamouda, R.; Sabahy, A.; Abbas, W.; Morsy, O.M. Biochar Production under Different Pyrolysis Temperatures with Different Types of Agricultural Wastes. Sci. Rep. 2024, 14, 2625. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science & Technology; Earthscan: London, UK, 2009; p. 448. [Google Scholar]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Varkolu, M.; Gundekari, S.; Omvesh; Palla, V.C.S.; Kumar, P.; Bhattacharjee, S.; Vinodkumar, T. Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts 2025, 15, 243. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility: A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd_Allah, E.F.; et al. Biochar Production and Characteristics, Its Impacts on Soil Health, Crop Production, and Yield Enhancement: A Review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Chen, G.; Gui, J.; Qin, C.-X.; Huang, D.-L.; Liao, F.; Yang, L.; Yang, L. Evaluating the impact of biochar on biomass and nitrogen use efficiency of sugarcane using 15N tracer method. Front. Agron. 2022, 4, 989993. [Google Scholar] [CrossRef]
- El-Naggar, A.; Awad, Y.M.; Tang, X.-Y.; Liu, C.; Niazi, N.K.; Jien, S.-H.; Tsang, D.C.W.; Song, H.; Ok, Y.S.; Lee, S.-S. Biochar influences soil carbon pools and facilitates interactions with soil: A field investigation. Land Degrad. Dev. 2018, 29, 2162–2171. [Google Scholar] [CrossRef]
- Luo, X.; Liu, G.; Xia, Y.; Chen, L.; Jiang, Z.; Zheng, H.; Wang, Z. Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. J. Soils Sediments 2017, 17, 780–789. [Google Scholar] [CrossRef]
- Oladele, S.O. Changes in physicochemical properties and quality index of an Alfisol after three years of rice husk biochar amendment in rainfed rice–maize cropping sequence. Geoderma 2019, 353, 359–371. [Google Scholar] [CrossRef]
- Pandian, K.; Vijayakumar, S.; Abu Firnass Mustaffa, M.R.; Subramanian, P.; Chitraputhirapillai, S. Biochar—A sustainable soil conditioner for improving soil health, crop production and environment under changing climate: A review. Front. Soil Sci. 2024, 4, 1376159. [Google Scholar] [CrossRef]
- Badan Pusat Statistik Kabupaten Malang. Data Klimatologi Pos Karangkates; Badan Pusat Statistik Kabupaten Malang: Malang, Indonesia, 2023; Available online: https://malangkab.bps.go.id/id/statistics-table/2/NjQjMg==/data-klimatologi-pos-karangkates.html (accessed on 16 June 2025).
- Farni, Y.; Suntari, R.; Prijono, S. Utilization of Tithonia diversifolia and sugarcane leaves to improve soil properties and plant growth on a degraded sandy soil of Malang, East Java. J. Degrad. Min. Lands Manag. 2022, 9, 3227–3236. [Google Scholar] [CrossRef]
- Fauzan, M.N.; Dwiastuti, R.; Sujarwo; Kurniawan, S. Changes in soil characteristics and estimated cost on reclamation of former sand mining land. J. Degrad. Min. Lands Manag. 2022, 9, 3533–3543. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patra, B.R.; Podder, J.; Dalai, A.K. Synthesis of biochar from lignocellulosic biomass for diverse industrial applications and energy harvesting: Effects of pyrolysis conditions on the physicochemical properties of biochar. Front. Mater. 2022, 9, 870184. [Google Scholar] [CrossRef]
- Yadav, K.; Jagadevan, S. Influence of process parameters on synthesis of biochar by pyrolysis of biomass: An alternative source of energy. In Recent Advances in Pyrolysis; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Kachurina, O.M.; Zhang, H.; Raun, W.R.; Krenzer, E.G. Simultaneous determination of soil aluminum, ammonium- and nitrate- nitrogen using 1 M potassium chloride. Commun. Soil Sci. Plant Anal. 2000, 31, 893–903. [Google Scholar] [CrossRef]
- Indonesian Soil Research Institute. Petunjuk Teknis Analisis Kimia Tanah, Tanaman, Air dan Pupuk, 2nd ed.; Indonesian Soil Research Institute: Bogor, Indonesia, 2009. [Google Scholar]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of Nitrogen in Agriculture and Environment: Agronomic, Eco-Physiological and Molecular Approaches to Improve Nitrogen Use Efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Pereira, V.V.; Morales, M.M.; Pereira, D.H.; de Rezende, F.A.; de Souza Magalhães, C.A.; de Lima, L.B.; Marimon-Junior, B.H.; Petter, F.A. Activated Biochar-Based Organomineral Fertilizer Delays Nitrogen Release and Reduces N2O Emission. Sustainability 2022, 14, 12388. [Google Scholar] [CrossRef]
- Bi, H.; Xu, J.; Li, K.; Li, K.; Cao, H.; Zhao, C. Effects of Biochar-Coated Nitrogen Fertilizer on the Yield and Quality of Bok Choy and on Soil Nutrients. Sustainability 2024, 16, 1659. [Google Scholar] [CrossRef]
- Liu, Z.; He, T.; Cao, T.; Yang, T.; Meng, J.; Chen, W. Effects of Biochar Application on Nitrogen Leaching, Ammonia Volatilization and Nitrogen Use Efficiency in Two Distinct Soils. J. Soil Sci. Plant Nutr. 2017, 17, 515–528. [Google Scholar] [CrossRef]
- Lapushkin, V.M.; Lapushkina, A.A.; Torshin, S.P. Effect of Coated Urea and NPK-Fertilizers on Spring Wheat Yield and Nitrogen Use Efficiency. Braz. J. Biol. 2024, 84, e279269. [Google Scholar] [CrossRef] [PubMed]
- Gamage, A.; Basnayake, B.; De Costa, J.; Merah, O. Effects of Rice Husk Biochar Coated Urea and Anaerobically Digested Rice Straw Compost on the Soil Fertility, and Cyclic Effect of Phosphorus. Plants 2022, 11, 75. [Google Scholar] [CrossRef]
- Singh, B.; Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.; Xiao, H.; Xie, T.; Qin, S.; Hu, G. Effects of nitrogen reduction combined with organic materials on crop yield, photosynthetic characteristics, and product quality of corn-cabbage rotation system. Chin. J. Appl. Ecol. 2021, 32, 4391–4400. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, F.; Yang, J.; Zhen, X.; Li, Y.; Zhao, J.; Li, J.; Qian, B.; Yang, D.; Li, X. Photosynthetic Characteristics and Uptake and Translocation of Nitrogen in Peanut in a Wheat–Peanut Rotation System under Different Fertilizer Management Regimes. Plants 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, C.; Li, Q.; Ye, J.; Lin, Y.; Wang, Y.; Burton, D.L. Evaluating the effect of biochar rate and combination with fertilizer on the dynamics of soil nitrogen supply in tea plantation. Sci. Rep. 2025, 15, 3135. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Evans, C.C.; Spicer, M.D. Plant Nitrogen Assimilation: A Climate Change Perspective. Plants 2025, 14, 1025. [Google Scholar] [CrossRef]
- Sanagi, M.; Aoyama, S.; Kubo, A.; Lu, Y.; Sato, Y.; Ito, S.; Abe, M.; Mitsuda, N.; Ohme-Takagi, M.; Kiba, T.; et al. Low Nitrogen Conditions Accelerate Flowering by Modulating the Phosphorylation State of FLOWERING BHLH 4 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2022942118. [Google Scholar] [CrossRef]
- Ali, A.; Jabeen, N.; Farruhbek, R.; Chachar, Z.; Laghari, A.A.; Chachar, S.; Ahmed, N.; Ahmed, S.; Yang, Z. Enhancing Nitrogen Use Efficiency in Agriculture by Integrating Agronomic Practices and Genetic Advances. Front. Plant Sci. 2025, 16, 1543714. [Google Scholar] [CrossRef]
| Plant Height (cm) | BCU Fertilizer Dose | Thickness of Biochar Layer | Average | |
| B1 | B2 | |||
| N1 | 46.53 c | 32.33 d | 39.43 b | |
| N2 | 55.73 ab | 57.53 ab | 56.63 a | |
| N3 | 49.00 bc | 60.67 a | 54.83 a | |
| N4 | 31.40 d | 45.00 c | 38.20 b | |
| N5 | 29.40 d | 52.60 abc | 41.00 b | |
| Average | 42.41 b | 49.63 a | ||
| Average Number of Leaves | BCU Fertilizer Dose | Thickness of Biochar Layer | Average | |
| B1 | B2 | |||
| N1 | 6 b | 5 d | 5 cd | |
| N2 | 6 b | 7 ab | 6 ab | |
| N3 | 7 ab | 7 a | 7 a | |
| N4 | 5 d | 5 d | 5 d | |
| N5 | 6 b | 6 b | 6 bc | |
| Average | 6 | 6 | ||
| Stem Diameter (mm) | BCU Fertilizer Dose | Thickness of Biochar Layer | Average | |
| B1 | B2 | |||
| N1 | 17.53 cd | 11.33 f | 14.43 c | |
| N2 | 22.33 ab | 20.33 abc | 21.33 a | |
| N3 | 19.53 bc | 23.00 a | 21.27 a | |
| N4 | 12.80 f | 14.00 ef | 13.40 c | |
| N5 | 16.33 de | 20.53 ab | 18.43 b | |
| Average | 17.71 | 17.84 | ||
| Length of corn cob (cm) | BCU Fertilizer Dose | Thickness of Biochar Layer | Average | |
| B1 | B2 | |||
| N1 | 13.50 | 8.85 | 11.18 | |
| N2 | 15.57 | 14.83 | 15.20 | |
| N3 | 13.17 | 17.00 | 15.08 | |
| N4 | 13.10 | 12.17 | 12.63 | |
| N5 | 13.60 | 11.17 | 12.38 | |
| Average | 13.79 | 12.80 | ||
| Diameter of corn cob (mm) | BCU Fertilizer Dose | Thickness of Biochar Layer | Average | |
| B1 | B2 | |||
| N1 | 40.65 bcde | 37.20 de | 38.93 b | |
| N2 | 46.35 ab | 43.75 abc | 45.05 a | |
| N3 | 35.10 e | 47.55 a | 41.33 ab | |
| N4 | 42.45 abcd | 40.00 cde | 41.23 b | |
| N5 | 43.50 abc | 39.17 cde | 41.33 ab | |
| Average | 41.61 | 41.53 | ||
| Weight of corn cob (g/plant) | BCU Fertilizer Dose | Thickness of Biochar Layer | Average | |
| B1 | B2 | |||
| N1 | 104.00 abc | 42.50 c | 73.25 b | |
| N2 | 157.25 a | 122.50 ab | 139.88 a | |
| N3 | 69.50 bc | 163.25 a | 116.38 ab | |
| N4 | 112.00 abc | 67.33 bc | 89.67 b | |
| N5 | 122.00 ab | 72.33 bc | 97.17 ab | |
| Average | 112.95 | 93.58 | ||
| Yield (t/ha) | BCU Fertilizer Dose | Thickness of Biochar Layer | Average | |
| B1 | B2 | |||
| N1 | 5.57 cd | 1.76 e | 3.67 b | |
| N2 | 8.42 ab | 6.07 bc | 7.24 a | |
| N3 | 3.88 cde | 9.69 a | 6.78 a | |
| N4 | 5.20 cd | 2.85 de | 4.02 b | |
| N5 | 5.43 cd | 2.82 de | 4.13 b | |
| Average | 5.70 a | 4.64 b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shofiati, S.; Nugroho, G.A.; Kusuma, Z.; Kurniawan, S. Effect of Biochar-Coated Urea on Soil Nitrogen, Plant Uptake, and Sweet Corn Yield in Sandy Soil. Nitrogen 2025, 6, 61. https://doi.org/10.3390/nitrogen6030061
Shofiati S, Nugroho GA, Kusuma Z, Kurniawan S. Effect of Biochar-Coated Urea on Soil Nitrogen, Plant Uptake, and Sweet Corn Yield in Sandy Soil. Nitrogen. 2025; 6(3):61. https://doi.org/10.3390/nitrogen6030061
Chicago/Turabian StyleShofiati, Sa’adah, Gabryna Auliya Nugroho, Zaenal Kusuma, and Syahrul Kurniawan. 2025. "Effect of Biochar-Coated Urea on Soil Nitrogen, Plant Uptake, and Sweet Corn Yield in Sandy Soil" Nitrogen 6, no. 3: 61. https://doi.org/10.3390/nitrogen6030061
APA StyleShofiati, S., Nugroho, G. A., Kusuma, Z., & Kurniawan, S. (2025). Effect of Biochar-Coated Urea on Soil Nitrogen, Plant Uptake, and Sweet Corn Yield in Sandy Soil. Nitrogen, 6(3), 61. https://doi.org/10.3390/nitrogen6030061






