Nitrogen Fertilization and Glomus Mycorrhizal Inoculation Enhance Growth and Secondary Metabolite Accumulation in Hyssop (Hyssopus officinalis L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Extract Content
2.2. Chlorophyll Content
2.3. Determination of Total Phenolic Content
2.4. Determination of Total Flavonoid Content
2.5. Determination of Total Anthocyanin Content
2.6. Isolation and Identification of Phenolic Acids
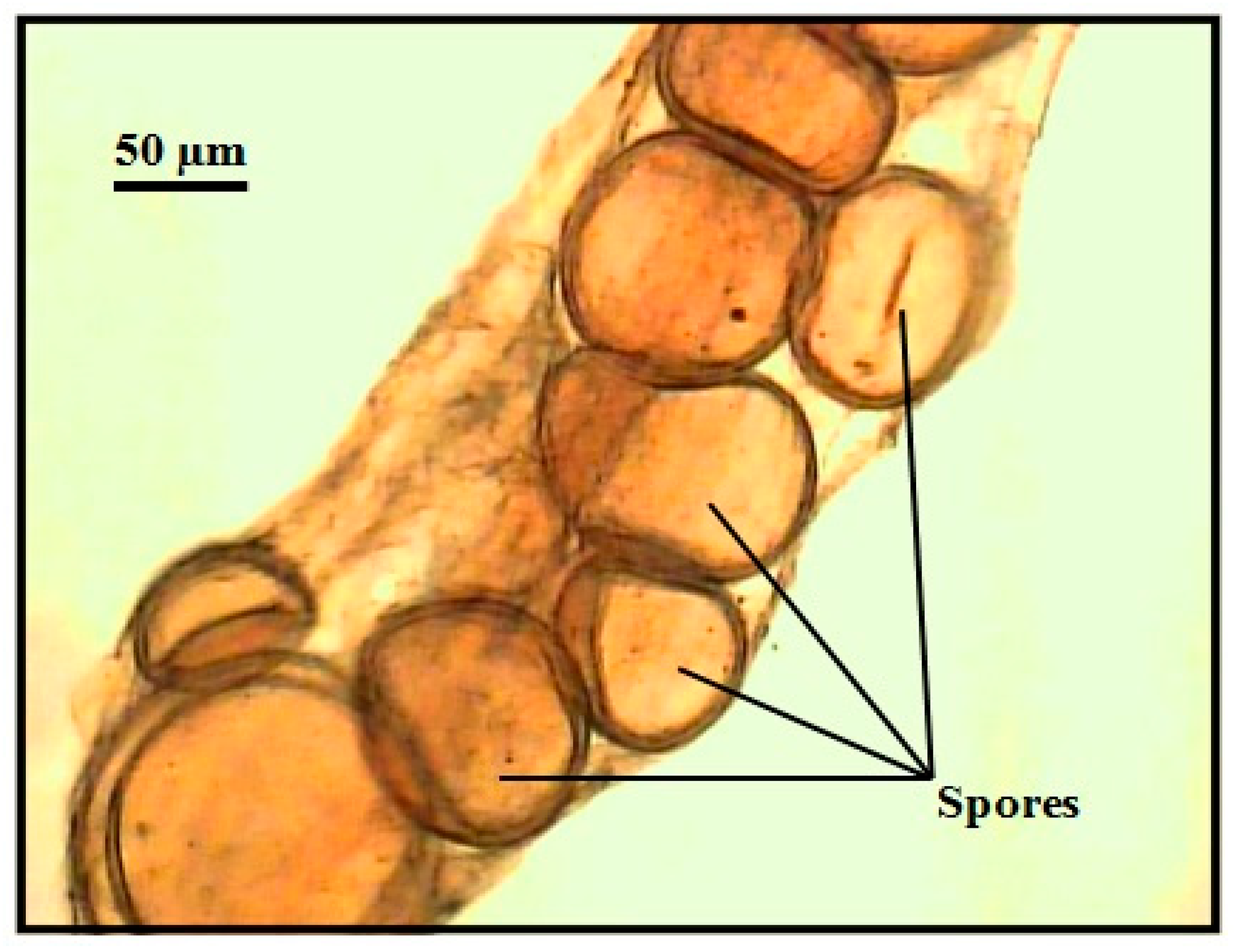
2.7. Assessment of Arbuscular Mycorrhizal Fungal Spore Extraction and Root Colonization
2.8. Shoot Nitrogen, Phosphorus and Potassium Content
2.9. Data Analysis
3. Results
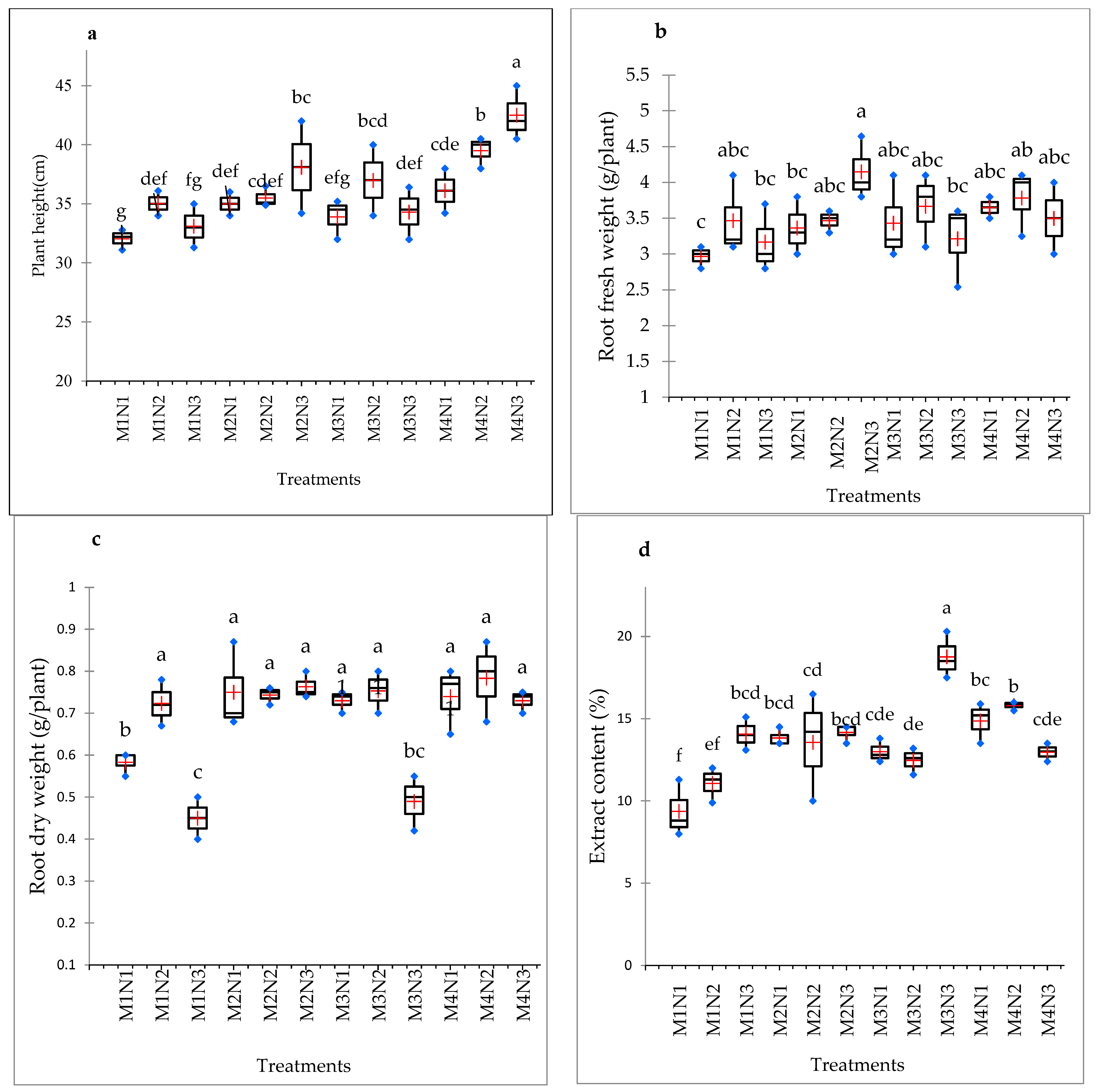
3.1. Fresh and Dry Biomass Production and Plant Height of Shoots
3.2. Fresh and Dry Weight of Roots
3.3. Extract Content
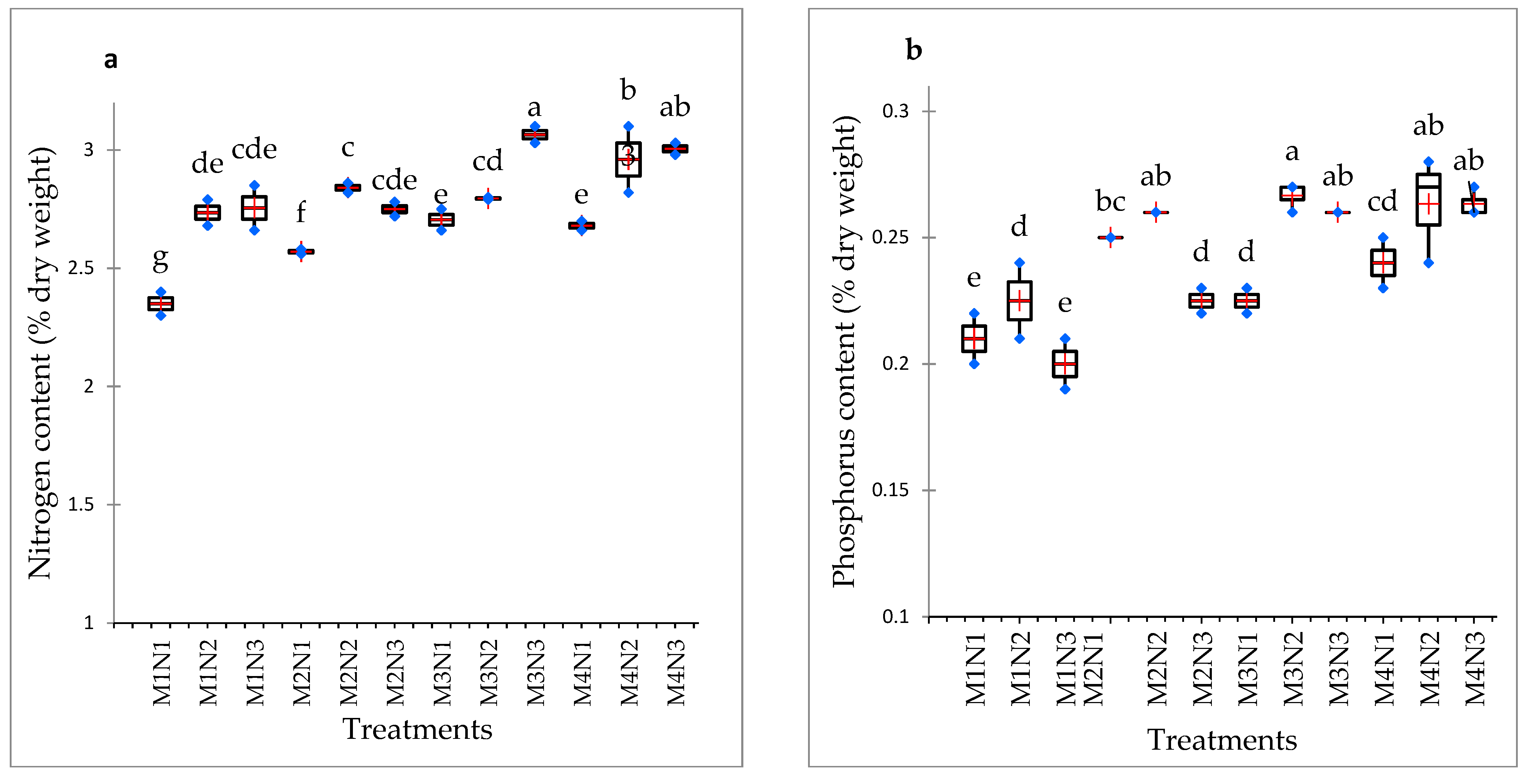
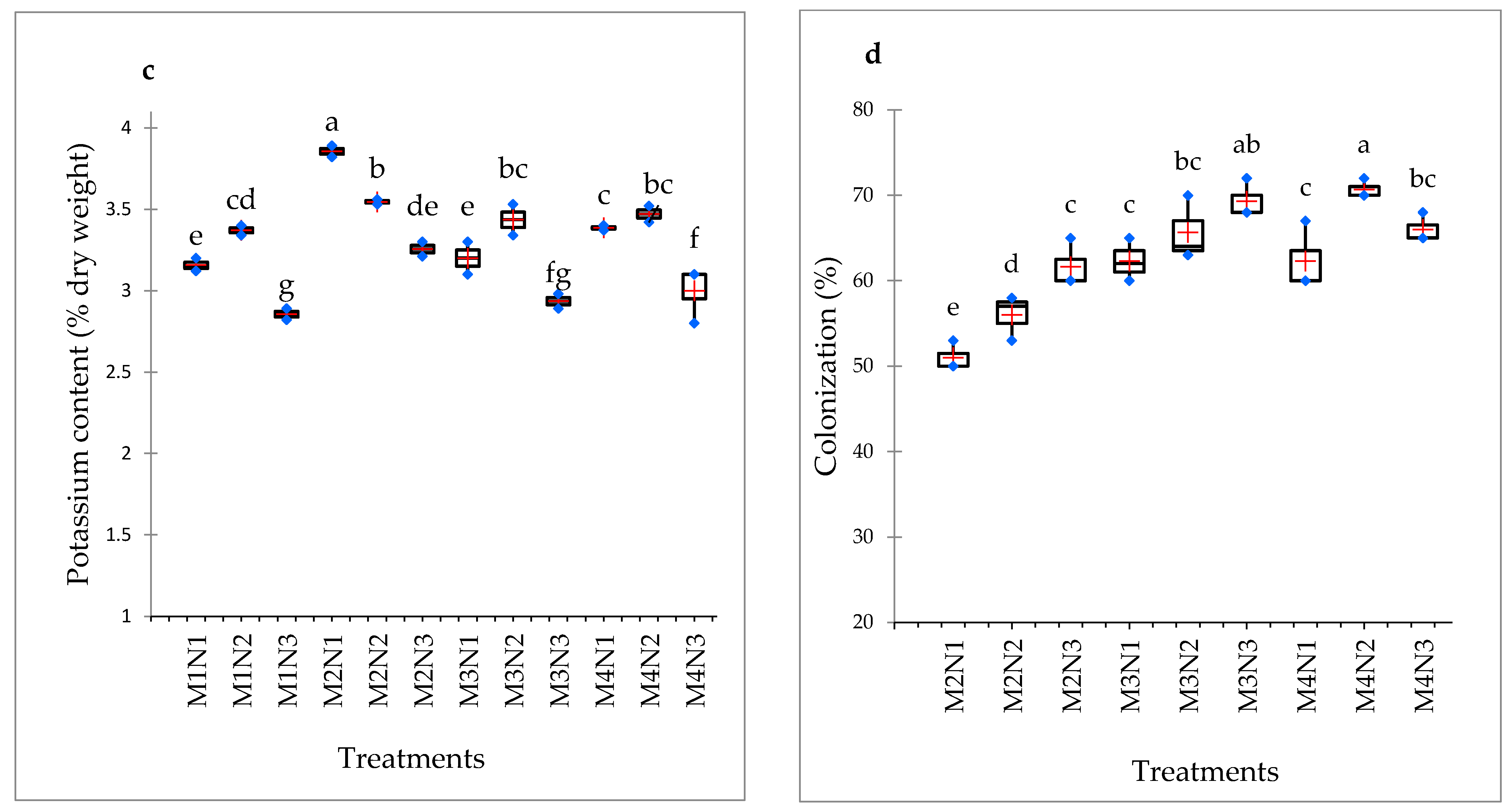
3.4. Nitrogen, Phosphorus and Potassium Content
3.5. Mycorrhizal Colonization Percentage
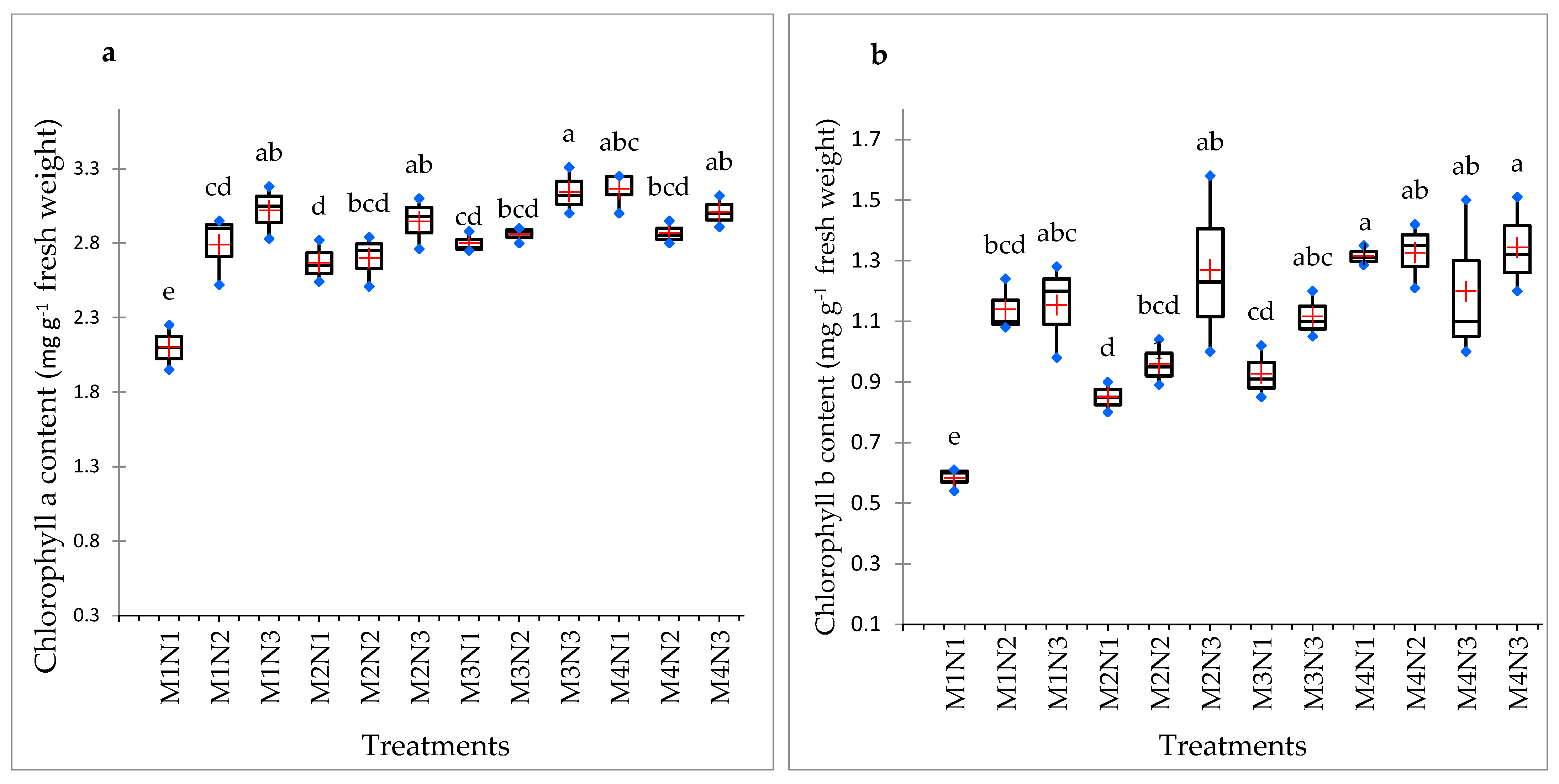
3.6. Chlorophyll a and b and Total Chlorophyll Content
3.7. Total Phenol and Flavonoid Content
3.8. Anthocyanin Content
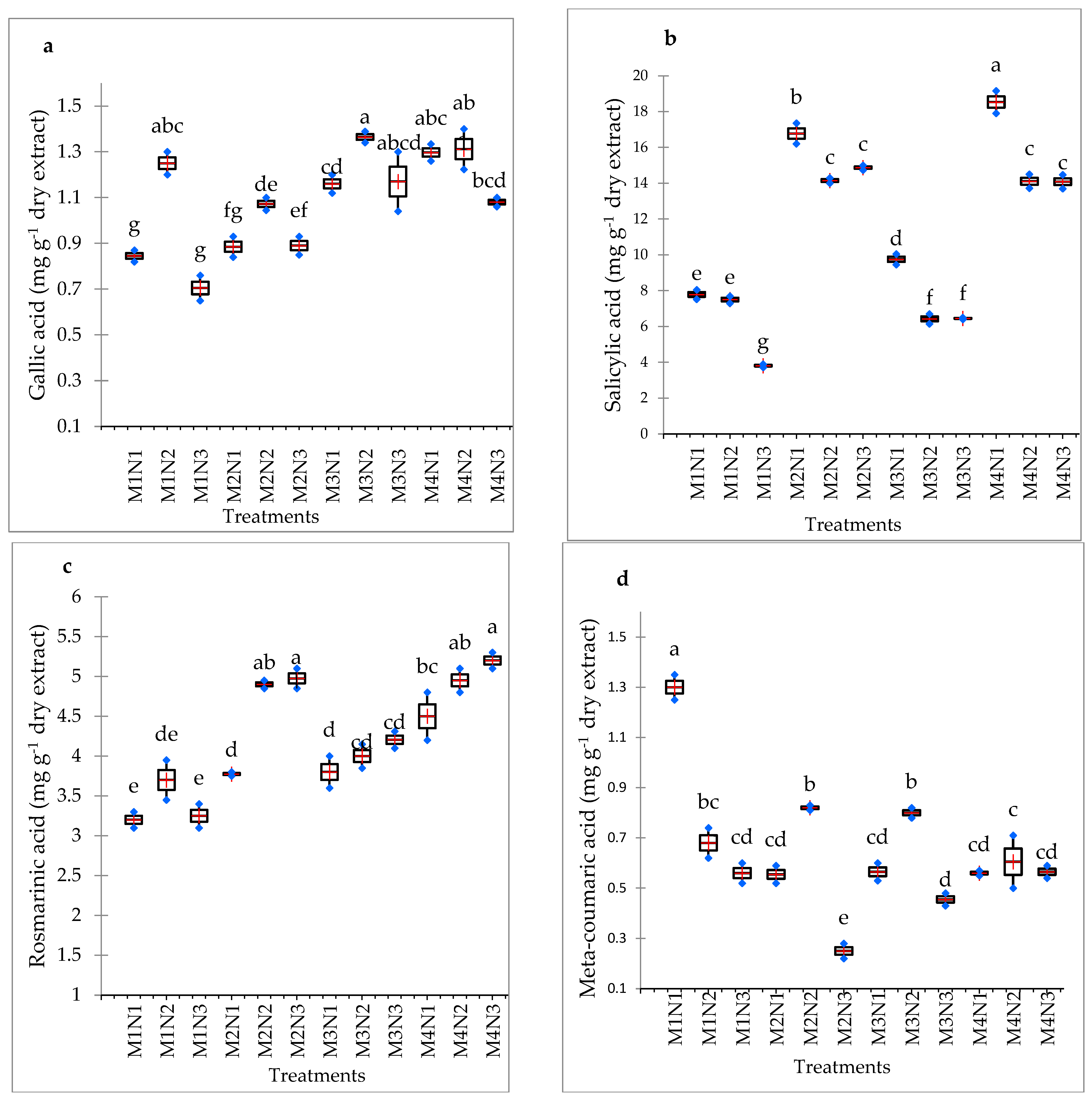
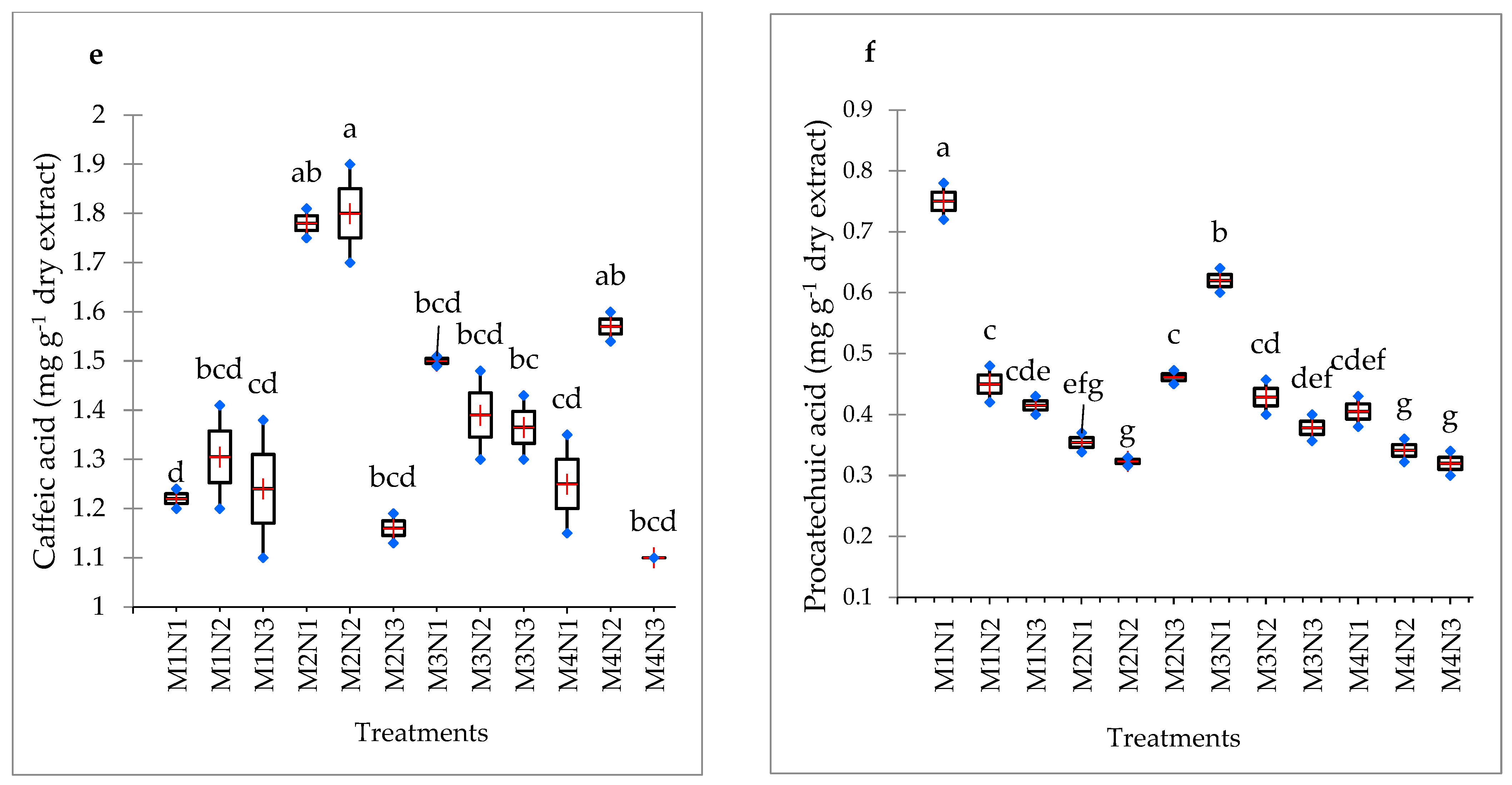
3.9. Phenolic Acid Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ochatt, S.; Alan, A.R.; Bhattacharya, A.; Hano, C.; Kiselev, K.V.; Marconi, P.L.; Otoni, W.C.; Park, S.Y.; Tang, K.X.; Weathers, P.J. Secondary metabolites: A boon from plants, the best chemist in nature: Preface from the editors. Plant Cell Tissue Organ Cult. 2022, 149, 1–6. [Google Scholar] [CrossRef]
- Selwal, N.; Rahayu, F.; Herwati, A.; Latifah, E.; Suhara, C.; Suastika, I.B.K.; Mahayu, W.M.; Wani, A.K. Enhancing secondary metabolite production in plants: Exploring traditional and modern strategies. J. Agric. Food Res. 2023, 14, 100702. [Google Scholar] [CrossRef]
- Hazrati, S.; Mousavi, Z.; Mollaei, S.; Sedaghat, M.; Mohammadi, M.; Pignata, G.; Nicola, S. Optimizing nitrogen fertilization to maximize yield and bioactive compounds in Ziziphora clinopodioides. Agriculture 2024, 14, 1690. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M.; Maggi, F.; Morshedloo, M.R. Application of combined fertilizers improves biomass, essential oil yield, aroma profile, and antioxidant properties of Thymus daenensis Celak. Ind. Crops Prod. 2018, 121, 434–440. [Google Scholar] [CrossRef]
- Aghaei, K.; Pirbalouti, A.G.; Mousavi, A.; Badi, H.N.; Mehnatkesh, A. Effects of foliar spraying of L-phenylalanine and application of bio-fertilizers on growth, yield, and essential oil of hyssop [Hyssopus officinalis L. subsp. angustifolius (Bieb.)]. Biocatal. Agric. Biotechnol. 2019, 21, 101318. [Google Scholar] [CrossRef]
- Fathiazad, F.; Hamedeyazdan, S. 2011. A review on Hyssopus officinalis L.: Composition and biological activities. Afr. J. Pharm. Pharmacol. 2011, 5, 1959–1966. [Google Scholar]
- Guerrini, A.; Sacchetti, G.; Echeverria Guevara, M.P.; Paganetto, G.; Grandini, A.; Maresca, I.; Menghini, L.; Di Martino, L.; Marengo, A.; Tacchini, M. Wild Italian Hyssopus officinalis subsp. Aristatus (Godr.) Nyman: From morphological and phytochemical evidences to biological activities. Plants 2021, 10, 631–647. [Google Scholar]
- Borrelli, F.; Pagano, E.; Formisano, C.; Piccolella, S.; Fiorentino, A.; Tenore, G.C.; Izzo, A.A.; Rigano, D.; Pacifico, S. Hyssopus officinalis subsp. aristatus: An unexploited wild-growing crop for new disclosed bioactives. Ind. Crops Prod. 2019, 140, 111594. [Google Scholar]
- Rosato, A.; Maggi, F.; Cianfaglione, K.; Conti, F.; Ciaschetti, G.; Rakotosaona, R.; Fracchiolla, G.; Clodoveo, M.L.; Franchini, C.; Corbo, F. Chemical composition and antibacterial activity of seven uncommon essential oils. J. Essent. Oil Res. 2019, 30, 233–243. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Frezza, C.; Conti, F.; Bini, L.M.; Giuliani, C.; Bramucci, M.; Quassinti, L.; Damiano, S.; Lupidi, G.; et al. Essential oil composition, polar compounds, glandular trichomes and biological activity of Hyssopus officinalis subsp. aristatus (Godr.) Nyman from central Italy. Ind. Crops Prod 2015, 77, 353–363. [Google Scholar] [CrossRef]
- Kizil, S.; Toncer, O.; Ipek, A.; Arslan, N.; Saglam, S.; Mahmood Khawar, K. Blooming stages of Turkish hyssop (Hyssopus officinalis L.) affect essential oil composition. Acta Agric. Scand. Sec B–Soil. Plant Sci. 2008, 58, 273–279. [Google Scholar]
- Jangi, F.; Ebadi, M.; Ayyari, M. Evaluation of qualitative characteristics of hyssop (Hyssopus officinalis L.) in infrared drying. Irani. J. Med. Aroma. Plants Res. 2019, 35, 876–887. [Google Scholar]
- Kazazi, H.; Rezaei, K.; Ghotb-Sharif, S.J.; Emam-Djomeh, Z.; Yamini, Y. Supercriticial fluid extraction of flavors and fragrances from Hyssopus officinalis L. cultivated in Iran. Food Chem. 2007, 105, 805–811. [Google Scholar] [CrossRef]
- Torikov, V.E. Medicinal Value of Vegetable, Fruit and Berry, Field Plants and Wild Plants; Publishing House of the Bryansk State Agricultural Academy: Bryansk, Russia, 2013. [Google Scholar]
- Sharifi-Rad, J.; Quispe, C.; Kumar, M.; Akram, M.; Amin, M.; Iqbal, M.; Koirala, N.; Sytar, O.; Kregiel, D.; Nicola, S.; et al. Hyssopus essential oil: An update of its phytochemistry, biological activities, and safety profile. Oxidative Med. Cell. Longev. 2022, 2022, 1155–1164. [Google Scholar] [CrossRef]
- Srivastava, A.; Awasthi, K.; Kumar, B.; Misra, A.; Srivastava, S. Pharmacognostic and pharmacological evaluation of Hyssopus officinalis L. (Lamiaceae) collected from Kashmir Himalayas, India. Pharmacogn. J. 2018, 10, 690. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Yeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A. Nitrogen and the future of agriculture: 20 years on. Ambio 2022, 51, 17–24. [Google Scholar] [CrossRef]
- Strzemski, M.; Płachno, B.J.; Mazurek, B.; Kozłowska, W.; Sowa, I.; Lustofin, K.; Załuski, D.; Rydzik, Ł.; Szczepanek, D.; Sawicki, J. Morphological, anatomical, and phytochemical studies of Carlina acaulis L. cypsela. Int. J. Mol. Sci. 2020, 21, 9230. [Google Scholar] [CrossRef]
- Du, L.; Zhong, H.; Guo, X.; Li, H.; Xia, J.; Chen, Q. Nitrogen fertilization and soil nitrogen cycling: Unraveling the links among multiple environmental factors, functional genes, and transformation rates. Sci. Total Environ. 2024, 951, 175561. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Skubij, N.; Dzida, K. Essential oil composition of summer savory (Satureja hortensis L.) cv. Saturn depending on nitrogen nutrition and plant development phases in raw material cultivated for industrial use. Ind. Crops Prod. 2019, 135, 260–270. [Google Scholar] [CrossRef]
- Skubij, N.; Dzida, K.; Jarosz, Z.; Pitura, K.; Jaroszuk-Sierocińska, M. Nutritional value of savory herb (Satureja hortensis L.) and plant response to variable mineral nutrition conditions in various phases of development. Plants 2020, 9, 706. [Google Scholar] [CrossRef]
- Wang, J.; Qin, X.; Xu, S.; Zhao, M.; Shu, P.; Xu, F. Nitrogen availability affects stem development and response to differential root-zone drought stress in Catalpa bungei. Environ. Exp. Bot. 2021, 186, 104429. [Google Scholar] [CrossRef]
- Cui, L.J.; Liu, X.Y.; Lin, J.; Shi, K.M. Effects of arbuscular mycorrhizal fungi on roots growth and endogenous hormones of Phoebe zhennan under salt stress. J. Nanjing For. Univ. 2020, 44, 119–124. [Google Scholar]
- Naderi, G.; Madani, H.; Farahani, E. The assessment effects of bio and chemical fertilizers on vegetative growth and essential oil of Hyssop (Hyssopus officinalis L.). Appl. Ecol. For. Sci. 2015, 1, 1–4. [Google Scholar] [CrossRef]
- Ghanbari-Odivi, A.; Fallah, S.; Carrubba, A. Optimizing Hyssop (Hyssopus officinalis L.) cultivation: Effects of different manures on plant growth and essential oil yield. Horticulturae 2024, 10, 894. [Google Scholar] [CrossRef]
- Pichon, N.A.; Cappelli, S.L.; Soliveres, S.; Mannall, T.; Nwe, T.Z.; Hölzel, N.; Klaus, V.H.; Kleinebecker, T.; Vincent, H.; Allan, E. Nitrogen availability and plant functional composition modify biodiversity-multifunctionality relationships. Ecol. Lett. 2024, 27, e14361. [Google Scholar] [CrossRef]
- Whitman, T.; Neurath, R.; Perera, A.; Chu-Jacoby, I.; Ning, D.; Zhou, J.; Nico, P.; Pett-Ridge, J.; Firestone, M. Microbial community assembly differs across minerals in a rhizosphere microcosm. Environ. Microbiol. 2018, 20, 4444–4460. [Google Scholar] [CrossRef]
- Mollavali, M.; Perner, H.; Rohn, S.; Riehle, P.; Hanschen, F.S.; Schwarz, D. Nitrogen form and mycorrhizal inoculation amount and timing affect flavonol biosynthesis in onion (Allium cepa L.). Mycorrhiza 2018, 28, 59–70. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef]
- Ferrol, N.; Azcón-Aguilar, C.; Pérez-Tienda, J. Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: An overview on the mechanisms involved. Plant Sci. 2019, 280, 441–447. [Google Scholar] [CrossRef]
- Barea, J.; Pozo, M.; López-Ráez, J.; Aroca, R.; Ruíz-Lozano, J.; Ferrol, N.; Azcón, R.; Azcón-Aguilar, C. Arbuscular Mycorrhizas and their Significance in Promoting Soil-Plant Systems Sustainability Against Environmental Stresses; CRC Press: Boca Raton, FL, USA, 2013; pp. 353–387. [Google Scholar]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and sustainable agriculture: Exploring arbuscular mycorrhizal fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Pozo, M.J.; Zabalgogeazcoa, I.; de Aldana, B.R.V.; Martinez-Medina, A. Untapping the potential of plant mycobiomes for applications in agriculture. Curr. Opin. Plant Biol. 2021, 60, 102034. [Google Scholar] [CrossRef]
- Bi, Y.; Zhang, J.; Song, Z.; Wang, Z.; Qiu, L.; Hu, J.; Gong, Y. Arbuscular mycorrhizal fungi alleviate root damage stress induced by simulated coal mining subsidence ground fissures. Sci. Total Environ. 2019, 652, 398–405. [Google Scholar] [CrossRef]
- Chen, W.; Mou, X.; Meng, P.; Chen, J.; Tang, X.; Meng, G.; Xin, K.; Zhang, Y.; Wang, C. Effects of arbuscular mycorrhizal fungus inoculation on the growth and nitrogen metabolism of Catalpa bungei CA Mey. under different nitrogen levels. Front. Plant Sci. 2023, 14, 1138184. [Google Scholar]
- de Assis, R.M.A.; Carneiro, J.J.; Medeiros, A.P.R.; de Carvalho, A.A.; da Cunha Honorato, A.; Carneiro, M.A.C.; Bertolucci, S.K.V.; Pinto, J.E.B.P. Arbuscular mycorrhizal fungi and organic manure enhance growth and accumulation of citral, total phenols, and flavonoids in Melissa officinalis L. Ind. Crops Prod. 2020, 158, 112981. [Google Scholar]
- Haji Mohammadi, A.; Zarghami, R.; Kashani, A.; Heydari Sharifabad, H.; Nour Mohammadi, G. The effect of mycorrhizas under water deficit conditions on some morphophysiological and biochemical traits in greenhouse cultivation of Stevia Tissue culture plantlets. J. Plant Res. 2023, 36, 16–28. [Google Scholar]
- Yadav, V.K.; Kumar, D.; Jha, R.K.; Bairwa, R.K.; Singh, R.; Mishra, G.; Singh, J.P.; Kumar, A.; Vinesh, B.; Yang, R.; et al. Effects of AMF inoculation and nitrogen application on nitrogen mineralization of coastal saline soil. J. Nanjing Univ. Sci. Technol. 2023, 45, 145–152. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- McDonald, S.; Prenzler, P.D.; Antolovich, M.; Robards, K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001, 73, 73–84. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food. Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Sutharut, J.; Sudarat, J. Total anthocyanin content and antioxidant activity of germinated colored rice. Int. Food Res. J. 2012, 19, 215–221. [Google Scholar]
- Babaei-Rad, S.; Mumivand, H.; Mollaei, S.; Khadivi, A. Postharvest UV-B and UV-C treatments combined with fermentation enhance the quality characteristics of Capparis spinosa L. fruit, improving total phenols, flavonoids, anthocyanins, phenolic acids, and antioxidant activity. Food Chem. 2025, 483, 144306. [Google Scholar] [CrossRef]
- Walker, C.; Schüßler, A.; Vincent, B.; Cranenbrouck, S.; Declerck, S. Anchoring the species Rhizophagus intraradices (formerly Glomus intraradices). Fungal Syst. Evol. 2021, 8, 179–201. [Google Scholar] [CrossRef]
- Vierheilig, H.; Schweiger, P.; Brundrett, M. An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol. Plant. 2015, 154, 423–434. [Google Scholar] [CrossRef]
- Novamsky, I.; Van Eck, R.; Van Schouwenburg, J.C.H.; Walinga, I. Total nitrogen determination in plant material by means of the indophenol blue method. Neth. J. Agric. Sci. 1974, 22, 3–5. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef]
- Mulyadi; Jiang, L. Combined application of Arbuscular Mycorrhizal Fungi (AMF) and nitrogen fertilizer alters the physicochemical soil properties, nitrogen uptake, and rice yield in a polybag experiment. Agriculture 2023, 13, 1364. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, S.X.; Anyia, A.O. Mycorrhizal inoculation and nitrogen fertilization affect the physiology and growth of spring wheat under two contrasting water regimes. Plant Soil 2016, 398, 47–57. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Al-Sadi, A.M.; Alharbi, S.A.; Datta, R.; Zuan, A.T.K. Biochar and arbuscular mycorrhizal fungi mediated enhanced drought tolerance in Okra (Abelmoschus esculentus) plant growth, root morphological traits and physiological properties. Saudi J. Biol. Sci. 2021, 28, 5490–5499. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, W.; Bi, N.; Guo, J.; Wang, L.; Zhao, J.; Zhang, J. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 2015, 88, 41–49. [Google Scholar] [CrossRef]
- Moradtalab, N.; Hajiboland, R.; Aliasgharzad, N.; Hartmann, T.E.; Neumann, G. Silicon and the association with an arbuscular-mycorrhizal fungus (Rhizophagus clarus) mitigate the adverse effects of drought stress on strawberry. Agronomy 2019, 9, 41. [Google Scholar] [CrossRef]
- Das, D.; Basar, N.U.; Ullah, H.; Salin, K.R.; Datta, A. Interactive effect of silicon and mycorrhizal inoculation on growth, yield and water productivity of rice under water-deficit stress. J. Plant Nutr. 2021, 44, 2756–2769. [Google Scholar] [CrossRef]
- Johnson, N.C.; Diane, L.; Corkidi, R.L.; Egerton-Warburton, L.M.; Allen, E.B. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 2003, 84, 1895–1908. [Google Scholar] [CrossRef]
- Treseder, K.K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef]
- Berghetti, A.L.P.; Araujo, M.M.; Tabaldi, L.A.; Turchetto, F.; Tassinari, A.; Bernardy, D.; Griebeler, A.M.; Barbosa, F.M.; Aimi, S.C.; Brunetto, G. Effects of nitrogen fertilization on the growth and on photochemical efficiency in plants of Handroanthus heptaphyllus. J. Plant Nutr. 2021, 44, 2464–2475. [Google Scholar] [CrossRef]
- Skudra, I.; Ruza, A. Effect of nitrogen and sulphur fertilization on chlorophyll content in winter wheat. Rural. Sustain. Res. 2017, 37, 29–37. [Google Scholar] [CrossRef]
- Chowdhury, T.; Chowdhury, A.H.; Oingyue, W.; Enyoh, C.E.; Wang, W.; Islam Khan, S. Nutrient uptake and pharmaceutical compunds of Aloe vera as in fluenced by integration of in organic fertilizer and poultry manure in soil. Heliyon 2021, 7, e07464. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, Q.; Shen, Y.K.; Yang, Q.; Zhang, J.B.; Wang, Y.H.; Song, X.Z. Effects of nitrogen deposition on arbuscular myorrhizal fungal colonization and glomalin-related soil protein of Chinese fir. Acta Ecol. Sin. 2021, 41, 194–201. [Google Scholar] [CrossRef]
- Pu, C.; Yang, G.; Li, P.; Ge, Y.; Garran, T.A.; Zhou, X.; Shen, Y.; Zheng, H.; Chen, M.; Huang, L. Arbuscular mycorrhiza alters the nutritional requirements in Salvia miltiorrhiza and low nitrogen enhances the mycorrhizal efficiency. Sci. Rep. 2022, 12, 19633–19641. [Google Scholar] [CrossRef]
- Yang, S.; Shi, Z.; Zhang, M.; Li, Y.; Gao, J.; Wang, X.; Liu, D. Stoichiometry of carbon, nitrogen and phosphorus in shrub organs linked closely with mycorrhizal strategy in northern China. Front. Plant Sci. 2021, 12, 687347. [Google Scholar] [CrossRef]
- Al-Karaki, G.N.; Williams, M. Mycorrhizal mixtures affect the growth, nutrition, and physiological responses of soybean to water deficit. Acta Physiol. Plant. 2021, 43, 75. [Google Scholar] [CrossRef]
- Azeez, J.O.; Van Averbeke, W.; Okorogbona, A.O.M. Differential responses in yield of pumpkin (Cucurbita maxima L.) and nightshade (Solanum retroflexum Dun.) to the application of three animal manures. Bioresour. Technol. 2010, 101, 2499–2505. [Google Scholar] [CrossRef]
- Sun, M.; Yuan, D.; Hu, X.; Zhang, D; Li, Y. Effects of mycorrhizal fungi on plant growth, nutrient absorption and phytohormones levels in tea under shading condition. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2006–2020. [Google Scholar]
- Gupta, M.L.; Prasad, A.; Ram, M.; Kumar, S. Effect of the vesiculararbuscular mycorrhizal (VAM) fungus Glumus fasciculatum on the essential oil yield related characters and nutrient acquisition in the crops of different cultivars of menthol mint (Mentha arvensis) under field conditions. Bioresour. Technol. 2002, 81, 77–79. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Chauhan, P.S.; DasGupta, S.M.; Seem, K.; Varma, A.; Staddon, W.J. Tripartite interactions among (Paenibacillus lentimorbus) NRRL B-3048, (Piriformospora indica) DSM 11827, and (Cicer arietinum L.). World J. Microbiol. Biotechnol. 2010, 26, 1393–1399. [Google Scholar] [CrossRef]
- Caravaca, F.; Diaz, E.; Barea, J.M.; Azcón-Aguilar, C.; Roldan, A. Photosynthetic and transpiration rates of Olea europaea subsp. sylvestris and Rhamnus lycioides as affected by water deficit and mycorrhiza. Biol. Plant. 2003, 46, 637–639. [Google Scholar]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd-Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Montoya-García, C.O.; Volke-Hallera, V.H.; Trinidad-Santosa, A.; Villanueva-Verduzco, C. Change in the contents of fatty acids and antioxidant capacity of purslane in relation to fertilization. Sci. Hortic. 2018, 234, 152–159. [Google Scholar] [CrossRef]
- Kheyri, Z.; Moghaddam, M.; Farhadi, N. Inoculation efficiency of different mycorrhizal species on growth, nutrient uptake, and antioxidant capacity of Calendula officinalis L.: A Comparative Study. J. Soil Sci. Plant Nutr. 2022, 22, 1160–1172. [Google Scholar] [CrossRef]
- Lazzara, S.; Militello, M.; Carrubba, A.; Napoli, E.; Saia, S. Arbuscular mycorrhizal fungi altered the hypericin, pseudohypericin, and hyperforin content in flowers of Hypericum perforatum grown under contrasting P availability in a highly organic substrate. Mycorrhiza 2017, 27, 345–354. [Google Scholar] [CrossRef]
- Hristozkova, M.; Gigova, L.; Geneva, M.; Stancheva, I.; Velikova, V.; Marinova, G. Influence of mycorrhizal fungi and microalgae dual inoculation on basil plants performance. Gesunde Pflanz. 2018, 70, 99–107. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Vojodi Mehrabani, L.; Badali, R.; Aazami, M.A.; Rasouli, F.; KaKaei, K.; Szczepanek, M. Cerium Oxide Salicylic Acid Nanoparticles’ (CeO2: SA-NPs) foliar application and in-soil animal manure use influence the growth and physiological responses of Aloe vera L. Agronomy 2022, 12, 731. [Google Scholar] [CrossRef]
- Rahimi, A.; Siavash Moghaddam, S.; Ghiyasi, M.; Heydarzadeh, S.; Ghazizadeh, K.; Popovic-Djordjevic, J. The influence of chemical, organic and biological fertilizers on agrobiological and antioxidant properties of Syrian Cephalaria (Cephalaria syriaca L.). Agriculture 2019, 9, 122. [Google Scholar] [CrossRef]
- Lingua, G.; Bona, E.; Manassero, P.; Marsano, F.; Todeschini, V.; Cantamessa, S.; Copetta, A.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria x ananassa var. Selva) in conditions of reduced fertilization. Int. J. Mol. Sci. 2013, 14, 16207–16225. [Google Scholar] [CrossRef]
- Rashidi, S.; Yousefi, A.R.; Pouryousef, M.; Goicoechea, N. Effect of three Species of mycorrhizal fungus on photosynthesis, growth and secondary metabolites content of Ipomoea purpurea L. Irani. J. Weed Sci. 2020, 16, 99–114. [Google Scholar]
- Zhang, Z.; Cai, B.; Guo, Y.; Na, T.; Guo, Y. The impact of different nitrogen levels on the tuber yield and anthocyanin synthesis of purple potatoes. Agriculture 2024, 14, 125. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Li, Y.Y.; Song, L.Q.; Zhao, L.L.; You, C.X.; Hao, Y.J. EIN3-LIKE1, MYB1, and ETHYLENE RESPONSE FACTOR3 act in a regulatory loop that synergistically modulates ethylene biosynthesis and anthocyanin accumulation. Plant Physiol. 2018, 178, 808–882. [Google Scholar] [CrossRef]
- Liang, J.; He, J. Protective role of anthocyanins in plants under low nitrogen stress. Biochem. Biophys. Res. Commun. 2018, 498, 946–953. [Google Scholar] [CrossRef]
- Engel, R.; Szabo, K.; Abranko, L.; Rendes, K.; Fuzy, A.; Takacs, T. Effect of arbuscular mycorrhizal fungi on the growth and polyphenol profile of marjoram, lemon balm, and marigold. J. Agric. Food Chem. 2016, 64, 3733–3742. [Google Scholar] [CrossRef]
- Chen, M.; Yang, G.; Liu, D.; Li, M.; Qiu, H.; Guo, L.; Huang, L.; Chao, Z. Inoculation with Glomus mosseae improves the growth and salvianolic acid b accumulation of continuously cropped Salvia miltiorrhiza. Appl. Sci. 2017, 7, 692. [Google Scholar] [CrossRef]
- Toussaint, J.P.; Smith, F.A.; Smith, S.E. Arbuscular mycorrhizal fungi can induce the production of phytochemicals in sweet basil irrespective of phosphorus nutrition. Mycorrhiza 2007, 17, 291–297. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Ormeño, E.; Fernandez, C. Effect of soil nutrient on production and diversity of volatile terpenoids from plants. Curr. Bioact. Compd. 2012, 8, 71–79. [Google Scholar]


| Item | Unit | Soil |
|---|---|---|
| Texture | - | Sandy loam |
| Organic carbon | (%) | 0.51 |
| EC | (ds/m) | 1.36 |
| pH | - | 8.20 |
| Total N | (%) | 0.05 |
| Available P | (mg kg−1) | 7.80 |
| Available K | (mg kg−1) | 162 |
| CaCO3 | (%) | 4.50 |
| Available Mg | (mg kg−1) | 9.84 |
| Available Fe | (mg kg−1) | 8.10 |
| Available Mn | (mg kg−1) | 6.22 |
| Available Zn | (mg kg−1) | 1.12 |
| Available Cu | (mg kg−1) | 1.05 |
| Available B | (mg kg−1) | 0.48 |
| Cation-exchange capacity (CEC) | (cmol kg−1) | 9.5 |
| Bulk density | (g cm−3) | 1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazrati, S.; Mohammadi, M.; Mollaei, S.; Ebadi, M.; Pignata, G.; Nicola, S. Nitrogen Fertilization and Glomus Mycorrhizal Inoculation Enhance Growth and Secondary Metabolite Accumulation in Hyssop (Hyssopus officinalis L.). Nitrogen 2025, 6, 60. https://doi.org/10.3390/nitrogen6030060
Hazrati S, Mohammadi M, Mollaei S, Ebadi M, Pignata G, Nicola S. Nitrogen Fertilization and Glomus Mycorrhizal Inoculation Enhance Growth and Secondary Metabolite Accumulation in Hyssop (Hyssopus officinalis L.). Nitrogen. 2025; 6(3):60. https://doi.org/10.3390/nitrogen6030060
Chicago/Turabian StyleHazrati, Saeid, Marzieh Mohammadi, Saeed Mollaei, Mostafa Ebadi, Giuseppe Pignata, and Silvana Nicola. 2025. "Nitrogen Fertilization and Glomus Mycorrhizal Inoculation Enhance Growth and Secondary Metabolite Accumulation in Hyssop (Hyssopus officinalis L.)" Nitrogen 6, no. 3: 60. https://doi.org/10.3390/nitrogen6030060
APA StyleHazrati, S., Mohammadi, M., Mollaei, S., Ebadi, M., Pignata, G., & Nicola, S. (2025). Nitrogen Fertilization and Glomus Mycorrhizal Inoculation Enhance Growth and Secondary Metabolite Accumulation in Hyssop (Hyssopus officinalis L.). Nitrogen, 6(3), 60. https://doi.org/10.3390/nitrogen6030060








