Abstract
This study assesses sediment toxicity in the historically contaminated Orbetello Lagoon (southern Tuscany) using Paracentrotus lividus embryo development bioassays. Elutriates from 15 sites were analysed for trace metals, organic matter, and ammonium. Despite elevated mercury concentrations, toxicity did not consistently correlate with metal levels. Instead, Principal Component Analysis (PCA) identified ammonium as a key driver of developmental toxicity, suggesting that it significantly influences both biological effects and metal bioavailability. These results demonstrate that ammonium, often overlooked, can confound sediment toxicity assessments and should be integrated into risk evaluation frameworks for coastal systems affected by legacy pollution.
1. Introduction
The Orbetello Lagoon, located at the southern end of Tuscany, comprises two in-terconnected basins, the west and east lagoons, covering a total area of 2525 hectares with an average depth of approximately one meter.
In the eastern portion of the western lagoon, the environmental quality of coastal wetlands is affected by the presence of the former SITOCO industrial plant, which operated until 1991 and produced granular fertilizers, generating critical byproducts such as nitric and sulfuric acid. The production of sulfuric acid involved the use of pyrite, whose roasting produced waste materials (pyrite ashes) rich in heavy metals and trace elements, including arsenic, lead, and cadmium [1].
Between 1873 and 1958, the eastern basin was heavily impacted by mining activity focused primarily on pyrite (FeS2), chalcopyrite (CuFeS2), and cinnabar (HgS), for the extraction of manganese and iron oxides used in the steel and metallurgical industries [1].
Due to contamination connected to its historical mining and industrial activities, particularly affecting the lagoon’s sediments, the Orbetello Lagoon has been classified as a national reclamation site (SIN) under Italian Law No. 179/2002.
Past environmental characterizations revealed severe contamination in the area adjacent to the industrial site, especially with metals such as As, Pb, Cu, Cd, Hg, and Zn, as well as PCBs, with contaminant levels gradually decreasing with distance from the site. Hydrocarbons and PAHs have also been detected near the lagoon’s sewage treatment plant [2,3].
In complex contamination scenarios such as this one, biological assays are commonly used to assess ecotoxicological risks and to support the selection of appropriate sediment management strategies. However, the accurate application and interpretation of these assays require careful consideration of potential confounding factors, such as ammonium—particularly when using sensitive species like Paracentrotus lividus [4].
The embryo development bioassay with the echinoderm P. lividus is extensively used to assess the ecotoxicological quality of brackish and marine environments, including sediment elutriates and water column samples, due to the high sensitivity of its larvae to both organic and inorganic contaminants [5,6]. Nonetheless, factors such as salinity and ammonium concentration can influence the test outcomes by either amplifying or masking the effects of toxic substances [4]. Ammonium, in particular, can alter the toxicity of trace metals through complexation reactions which reduce their bioavailability [7]. Additionally, its interaction with metals like Fe(III) and Mn(IV) can stimulate microbial processes, consequently affecting nitrogen removal and metal cycling, resulting in the modulation of environmental impacts [8]. With regard to Hg, complex interactions between ammonium and mercury toxicity have been reported in studies on the marine diatom Skeletonema costatum [9].
In this study, elutriates from sediments originating from the Orbetello Lagoon, known for significant mercury concentrations due to both natural geochemical anomalies [10,11] and anthropogenic activities [12], were tested using the P. lividus embryonic development bioassay.
This study tests the hypothesis that ammonium, in addition to mercury, plays a significant role in driving sediment toxicity in the Orbetello Lagoon. The main steps of the investigation are summarized in Supplementary Figure S1.
Our findings have direct implications for sediment management policies, suggesting that regulatory frameworks should account for both metallic and non-metallic toxicants when assessing sediment quality.
2. Materials and Methods
2.1. Sediment Collection
Superficial sediment samples were collected in February 2023 in the Orbetello lagoon (Figure 1) using an Ekman–Birge grab with a sampling area of 225 cm2 and depth of 15 cm. Sediments were sampled in fifteen sites along a contamination gradient of mercury according to previous ISPRA characterization [2]. Sediment samples were collected and processed following the procedures outlined in the U.S. EPA technical manual [13].
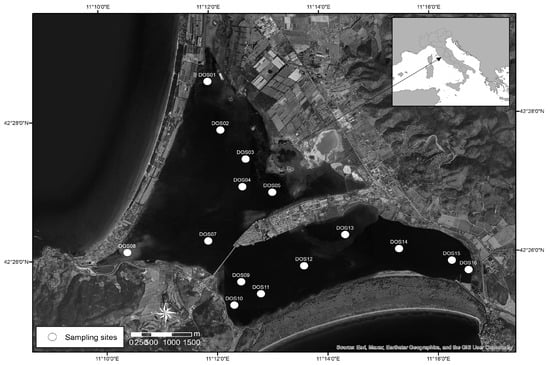
Figure 1.
Location of sampling sites in the study area of Orbetello Lagoon.
2.2. Elutriates Preparation
Elutriates were prepared in a 2 L capped polyethylene vessel according to the ASTM protocol [14], as described in Sartori et al. [15].
2.3. Chemical Analyses
The concentrations of heavy metals (As, Cd, Cr, Cu, Ni, Pb, Zn) in sediment samples were determined using inductively coupled plasma optical emission spectrometry (ICP-OES, Thermo iCAP 7200, Agilent, Santa Clara, CA, USA), following the protocol outlined by ICRAM [16].
Sediment samples (~0.5 g) were first dried at 35 °C and homogenized using manual grinding in an agate mortar. Acid digestion was performed using a microwave digestion system (ETHOS UP, Milestone, Sorisole, BG, Italy). For initial mineralization, 3 mL of HCl and 9 mL of HNO3 were added to each sample and allowed to react at room temperature for 20 min, followed by microwave-assisted digestion.
Subsequently, 2 mL of HF was added, and the mixture was left to rest for another 20 min before undergoing a second digestion cycle. After cooling, 30 mL of saturated H3BO3 solution was added, and the final volume was adjusted to 50 mL with ultrapure water in a calibrated volumetric flask.
All analyses were performed in duplicate. Blank tests were run under identical conditions. The certified reference material PACS-2 (marine sediment) was used to evaluate analytical accuracy (recoveries between 87% and 105%) and precision (relative standard deviation [RSD] routinely <8%, n = 3). Duplicates and reagent blanks were prepared and analyzed for all samples to assess reproducibility (better than 15% for all elements except Hg and Cd) and to determine the detection limits.
Mercury concentrations were determined separately using a direct mercury analyzer (DMA-80 atomic absorption spectrophotometer, Milestone, Sorisole, BG, Italy), in accordance with the US EPA Method 7473 [17]. Analytical accuracy was verified using the certified reference material PACS-3 (marine sediment), with an estimated error of 5%. Sample duplicates showed a reproducibility better than 12%.
Polycyclic Aromatic Hydrocarbons (PAHs) were analyzed using gas chromatography and mass spectrometry. Sample preparation was performed using pressurized fluid extraction (PFE) according to EPA 3545A [18]. The extracts were then analyzed by gas chromatography coupled with mass spectrometry (GC/MS), following EPA 8270E [19] protocols. Quantifications of polychlorinated biphenyl (PCBs) were conducted according to the EPA 1668C [20].
The sulfide content in sediment samples is determined by acid extraction, where hydrogen sulfide (H2S) is released upon treatment with hydrochloric acid (HCl). The released H2S is then captured in a zinc acetate solution, forming zinc sulfide. This is followed by reaction with the Cline reagent (methylene blue method), producing a blue-colored complex. The intensity of the color is measured spectrophotometrically at approximately 670 nm, and the sulfide concentration is quantified against a calibration curve.
The ammonium (NH4+) content in the elutriate was determined using Hach Lange kit LCK304 and LCK303 (Hach Lange GmbH, Düsseldorf, Germany) with a spectrophotometer Hach Lange DR3900 (Hach Lange GmbH, Düsseldorf, Germany).
2.4. Organic Matter Content
The organic matter content was determined using the Loss on Ignition (LOI) methodology [21]. Briefly, the sediment sample was subjected to three phases of combustion at increasing temperatures. Approximately 7 g of sediment was dried at 105 °C in weighted crucibles, for 24 h. Then, the sample was dried in a muffle, at 160 °C and 400 °C, for 6 and 4 h, respectively. After each step, the weight was determined. The content of organic matter was expressed by the following relation:
Organic Matter (%) = (Net weight 160 °C − Net weight 400 °C) × 100/(Net weight 105 °C)
2.5. Paracentrotus Lividus Embryo-Development Test
Specimens of P. lividus were collected from rocky shores along the southern coast of Livorno (43°25.6020′ N, 10°23.7800′ E) and immediately transported to the laboratory in a refrigerated container. P. lividus was selected due to its high sensitivity to ammonium, which has been shown to induce significant developmental toxicity even at low concentrations, making it a reliable indicator species for detecting ammonium-related effects in marine sediments [4]
The embryo development bioassay with P. lividus was performed according to Sartori et al. [4]. In brief, sea urchins were induced to spawn through the injection of 0.5 mL of 0.5 M KCl (Sigma-Aldrich, St. Louis, MO, USA) through the peristome. Gametes were collected from a minimum of three males and three females for each experimental trial. Sperm was collected dry using a Pasteur pipette, pooled, and stored at 4 °C until further use. Oocytes were collected in a 100 mL beaker filled with 0.22 μm filtered seawater (FSW), assessed for maturity under an Olympus GX53 inverted microscope (Olympus, Tokyo, Japan), and subsequently pooled in a 500 mL beaker. The egg suspensions were diluted to achieve a final concentration of 1000 oocytes/mL. After confirming sperm motility, 10 μL of sperm was gently mixed with the egg suspension. The success of fertilization, indicated by the formation of a fertilization membrane following the cortical reaction, was verified by examining an aliquot of embryos under an Olympus GX53 inverted microscope (Olympus, Tokyo, Japan). Post-fertilization, 1 mL of the embryo suspension (containing 1000 embryos) was exposed to test solutions, bringing the final volume to 10 mL. Experiments were conducted in polystyrene six-well plates (Corning Life Sciences, Tewksbury, MA, USA). The suitability of the biological material was validated by analyzing untreated negative controls (0.22 μm FSW collected from the sea urchin sampling site) and positive controls exposed to Cu(NO3)2·3H2O (Sigma-Aldrich, St. Louis, MO, USA). For the reference value, embryos in the positive control group were exposed to nominal copper concentrations of 10, 20, 30, 40, and 50 μg/L. Each test solution was prepared in three replicates. Embryos were incubated in the dark at 18 °C for 72 h and then fixed with 1% Lugol’s solution. The percentage of normally developed plutei was determined by counting 200 embryos per replicate under a Leica DMI 3000B inverted light microscope (Milan, Italy). Normal and abnormal P4 larvae were classified following the criteria described by Pagano et al. [22]. Fully developed larvae with normal morphology were considered “normal,” while specimens identified as retarded gastrulae, pre-gastrulae, prism stages, or malformed plutei—with skeletal and/or digestive system abnormalities—were classified as “abnormal.” The results were deemed acceptable if the percentage of normal plutei exceeded 80% in the negative control tests [23].
Fresh standard solutions of copper (Cu) were prepared immediately prior to analysis and subsequently used in the bioassays. Triplicate samples were prepared for each Cu concentration and analyzed using a graphite furnace atomic absorption spectrometer (AAS-GF, Varian Spectra AA-220 Z, Agilent Technologies, Torino, Italy), following the procedures outlined in EPA Method 7010 [23]. This analysis was performed to verify the consistency between the nominal and actual Cu concentrations in the prepared solutions. No matrix modifiers were utilized in the process. The determination was based on a calibration curve ranging from 10 to 100 μg/L. To ensure quality control, standard solutions obtained from Sigma-Aldrich were analyzed, with recovery rates consistently maintained between 90% and 110%.
2.6. Statistical Data Analysis
Due to the complexity of variables in this study, the concentration of metals in sediment, ammonium and the percentage of abnormal plutei after exposure to elutriates were analyzed with a Principal Component Analysis (PCA), after the normalization of data. As previously reported by different authors [24,25], the threshold for the acceptance of this analysis was 60% of the cumulative variance explained by the first two components. In order to enhance the interpretability of results, components were rotated using the Varimax method [26,27] with Kaiser normalization during the rotation to standardize the length of the factor loading vectors. All statistical permutations were conducted with SPSS v. 30.
To evaluate the toxic effects of copper and sediment elutriates on sea urchin larval development, ECx values (% effective concentrations causing abnormal larvae) were calculated. These values, along with their 95% confidence intervals, were estimated using the MOSAIC web interface for ecotoxicological statistical analysis [28], which is based on the R package morse (R version 4.5.0) [29].
3. Results
Given the low contamination levels in the sediments analyzed, the concentrations of Hydrocarbons, PAHs and PCBs are not shown in this section but are available in the Appendix A.1 (Table A1).
The sediment organic matter content varies from 0.9 (DOS 04) to 12.1% (DOS 05). Granulometric analysis revealed that the majority of samples were silt-dominated, with the silt content ranging from 37.21% to 100%. Four samples (DOS 05, DOS 08, DOS 10, DOS 12) showed a sand content above 40%, while gravel was absent in all cases (Table A2).
The metal concentrations measured in sediments are summarized in Table 1, along with sulfides, the concentration of ammonium (mg/L) and the EC20/EC50 values (%) obtained from the embryotoxicity test with P. lividus.

Table 1.
Concentrations of trace metals (mg/kg, dry weight) and organic matter (%, dry weight) measured in sediments from the Orbetello Lagoon. Ammonium concentrations (NH4+, mg/L) were determined in elutriates prepared from the same sediments. EC20 and EC50 values (%) obtained from P. lividus embryo development bioassays are also reported. Concentrations are expressed as mean ± SD (n = 3). Limit of quantification (LOQ) for trace metals analyzed are listed in table.
The chromium (Cr) levels ranged from 19.1 mg/kg at station DOS 05 to 66.9 mg/kg at DOS 08, with the highest values detected at DOS 08 (66.9 mg/kg) and DOS 07 (52.1 mg/kg).
Copper (Cu) concentrations showed marked variability among stations, with the highest values recorded at DOS 08 (66.2 mg/kg), followed by DOS 05 (16.3 mg/kg). DOS 08 also exhibited elevated concentrations of nickel (Ni), with a value of 31.1 mg/kg, and mercury (Hg), at 1.14 mg/kg.
Mercury concentrations across the Orbetello Lagoon ranged from 0.14 mg/kg (DOS 05) to 9.29 mg/kg (DOS 16). Notably, Hg levels exceeded the L2 (0.8 mg/kg) threshold value defined by the Italian Ministerial Decree 173/2016 [30] in several samples, including DOS 07, DOS 08, DOS 09, DOS 10, DOS 11, DOS 12, DOS 13, DOS 14, and DOS 15.
Arsenic (As) concentrations were below the limit of quantification (LOD) in all sediment samples analyzed.
Zinc (Zn) concentrations were remarkable in multiple stations, particularly in DOS 02, DOS 03, DOS 04, DOS 07, DOS 08, DOS 10, and DOS 12, with the highest concentration detected in DOS 04 (235.4 mg/kg), while the lowest was observed at DOS 05 (74.3 mg/kg).
The concentrations of ammonium measured in the elutriates ranged from 0.115 to 6.99 mg/L, as observed in samples DOS 05 and DOS 07, respectively. However, numerous elutriates exhibited NH4+ concentrations exceeding 1 mg/L, with the exception of DOS 01 and DOS 03, which did not report detectable concentrations.
Regarding the embryo development test with P. lividus, the percentage of normal-developed plutei respected the acceptability criteria, with a percentage in negative control (0.22 FSW) of 85%; meanwhile, the EC50 value obtained with the reference toxicant (Cu(NO3)2 × 3H2O) was 24.48 (23.57–25.80) μg/L. The latter falls within the range of values in the laboratory control chart (15.21–62.53 μg/L) and agrees with data reported in the literature for this species with this reference toxicant [31,32].
The embryonic development test with P. lividus indicated a complete absence of toxicity (EC20 > 90) in samples DOS 03, DOS 04, DOS 05, DOS 08, DOS 11, DOS 12, DOS 13 and DOS 14. Conversely, the elutriates from samples DOS 07, DOS 09, and DOS 15 exhibited the highest toxicity, with EC50 values ranging from 12.70 (9.61–15.60) for sample DOS 07, to 15.80 (11.90–19.80) for sample DOS 09, and up to 51.64 (45.28–60.36) for sample DOS 15.
Moderate toxicity was also observed in the remaining samples, with EC20 values ranging from 74.05 (40.26–91.88), detected in sample DOS 16, to 88.64 (81.26–94.48), as observed in sample DOS 01.
The PCA (Table 2) identified two principal components explaining 77.89% of the total variance. PC1 (64.25%) is strongly associated with most trace metals (e.g., Cr, V, Ni, Al, Cd) whereas PC2 (13.64%) is mainly influenced by ammonium and the percentage of abnormal plutei after the embryotoxicity assay, suggesting a separate factor linked to organic enrichment and potential biological stress.

Table 2.
Total variance explained by the first two components in the PCA. Rotated component matrix obtained with Varimax rotation and Kaiser normalization to standardize the length of the factor loading vectors.
Mercury shows a moderate association with PC2, separating itself from the rest of metals in this study (Figure 2).
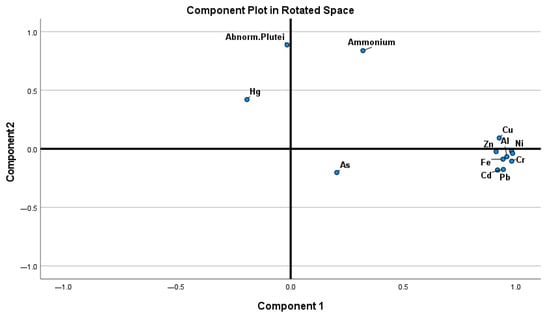
Figure 2.
Principal Component Analysis of metals, ammonium in sediments from Orbetello lagoon and toxicity measured as percentage of abnormal plutei of P. lividus (Abnorm. Plutei).
4. Discussion
In this study, only superficial sediments (0–15 cm) were collected, and both chemical and ecotoxicological analyses were conducted on whole sediments, in line with U.S. EPA guidelines [13]. Sampling was performed during the winter season to avoid the summer dystrophic crises characteristic of the Orbetello Lagoon [33,34], which are known to increase organic matter degradation and ammonium concentrations [35,36], potentially masking the specific effects of sediment-bound contaminants.
The analysis of organic contaminants did not detect significant concentrations of PAHs in the sediments of the investigated area (see Appendix A.1, Table A1). Similarly, PCB concentrations (see Appendix A.1, Table A1) were well below the probable effect level values (PELs) reported in the Canadian Sediment Quality Guidelines [37,38] and the L1 thresholds defined in Italian Decree 173/2016 [30]. Given the high hydrophobicity and molecular weight of PCBs and high-molecular-weight PAHs (HPAHs), these compounds tend to strongly bind to organic matter in sediments due to their elevated Kow, limiting their mobility in water under typical elutriate conditions [39,40,41]. For this reason, we have chosen not to discuss these data further and instead report their concentrations solely in the Appendix A.1 (Table A1).
The elevated zinc concentrations detected in Orbetello Lagoon sediments are consistent with the geochemical anomalies previously reported along the Tuscan continental shelf [42], characterized by an estimated background value for sediments from southern Tuscany of 182 mg/kg. However, anthropogenic sources like agricultural runoff, wastewater from aquaculture operations, and sewage discharges likely contribute to the observed enrichment of trace metals, including zinc [36,43]. Additionally, the historical activity of the ex-SITOCO fertilizer plant (1908–1985) likely exacerbated metal loading through the release of sulfuric acid, copper sulfate, and pyrite-rich ashes containing Zn, Cd, Pb, and As [1].
In contrast, the arsenic levels in this study were generally low: with the exception of station DOS 13 (0.9 mg/kg), the arsenic concentrations in all sediment samples remained below the analytical limit of quantification (LOQ = 0.0441 mg/kg), suggesting limited mobility or the absence of local sources.
Cadmium concentrations, although detectable, were consistently below the toxicity thresholds established for sea urchin embryos. Indeed, in the literature, the EC50 values for sea urchin larvae range between 2.06 and 9.24 mg/kg [44,45,46], significantly higher than those observed in Orbetello sediments; this scenario indicates a low risk of acute cadmium toxicity under the current conditions.
The mercury concentrations in sediments fell within the range previously reported by other authors [11,12,47] in the same area (0.15–4.55 mg/kg, with a peak of 14.9 mg/kg). Elevated Hg levels in sediments from Ansedonia are likely natural, reflecting geogenic anomalies in southern Tuscany [48] and sedimentological processes influencing mercury accumulation [49,50]. Past mining activities (1873–1958) for pyrite, chalcopyrite, and cinnabar in the eastern basin [11] likely contributed further to mercury enrichment.
In 87% of the samples, organic matter was above 5% and appeared to be a key modulator in the toxicological response observed. Organic ligands chelate more than 99% of metals in seawater, reducing their bioavailability and toxicity [51]. Besser et al. [52] demonstrated that the cadmium and copper toxicity decreased significantly in sediments amended with a high humus content (8.9% TOC). Similarly, DOC has been shown to attenuate trace element toxicity through complexation and reduced mobility [53,54,55]. Functional groups such as carboxyl, phenolic, and amino moieties dominate metal binding, though this is highly sensitive to pH and ionic strength [56,57]. Moreover, Damikouka et al. [58], when studying the changes in heavy metal partitioning to binding phases in contaminated marine sediments and the release of metals to the environment in sediment from the Port of Piraeus, found that the fraction of heavy metals mobilized from sediment to water during elutriate tests (Modified Elutriate Test, [59]) is typically <0.5, under both oxic and anoxic conditions. It is therefore plausible that, with the low concentrations of metals measured in sediments, most of the metals present did not result in a toxic response. This conclusion finds confirmation in the separation of metals and toxicity on the two opposite components of the PCA (Figure 2). Apart from arsenic, which was lower than the quantification level, the only contaminant which showed a different behavior was mercury.
Mercury bioavailability and toxicity are likewise influenced by dissolved organic matter (DOM), especially via sulfur-binding sites that stabilize Hg species and suppress methylmercury (MeHg) formation [60]. DOM has been shown to mitigate MeHg toxicity in early life stages of fish [61]. In our study, mercury concentrations did not always correlate with toxicity. Sample DOS 16, with the highest mercury concentration (9.4 mg/kg), exhibited only mild toxicity. In contrast, sample DOS 15, with a lower mercury concentration (3.68 mg/kg), showed high toxicity (EC50 = 51.64 [45.28–60.36]). This inconsistency suggests that factors beyond total metal concentration, such as metal speciation and sediment matrix composition, play a critical role in modulating toxicity.
The lower toxicity observed in DOS 16 may therefore be explained by the higher organic matter content and the presence of sulfide in this sample, while the higher toxicity of DOS 15 could reflect its lower organic matter concentration (5.4 g/kg). The three major contributors to component 2 were abnormal plutei, mercury and ammonium, contributing, respectively, with 0.420, 0.838 and 0.887 loadings in the variance measured. Hence, ammonium contributed more than mercury to the measured toxicity and may have acted as confounding toxicants in elutriates (Figure 3).

Figure 3.
Concentration of mercury (mg/Kg) measured in sediments and ammonium concentrations (NH4+; mg/L) and recorded in elutriates prepared from the same sediments.
Beyond chemical composition, sediment texture also appeared to influence toxicity patterns. Samples with a higher sand content (DOS 05, DOS 08, DOS 12) showed no toxicity, and DOS 10 exhibited only mild effects. This suggests that coarser sediments are generally less toxic, likely due to reduced contaminant retention and lower overall bioavailability [62,63].
Ammonium released during sediment resuspension can influence ecotoxicological outcomes, especially in embryotoxicity assays with P. lividus. The confounding role of ammonium in this study likely stems from both its direct embryotoxicity and its influence on mercury speciation and bioavailability. P. lividus is highly sensitive to ammonium, with EC50 values as low as 0.81 mg/L [4], and several of our samples exceeded this threshold. Additionally, ammonium can alter redox conditions and stimulate microbial activity, potentially enhancing mercury methylation or stabilizing less bioavailable forms [10]. These dual mechanisms—direct toxicity and speciation effects—help explain why mercury concentrations alone did not consistently predict toxicity outcomes [9].
This suggests that ammonium, although a product of natural organic matter degradation, may function as a pollutant in ecotoxicological terms when present above critical thresholds.
Martínez-Gómez et al. [64] reported widespread embryotoxic effects in sea urchin (P. lividus) embryos exposed to sediments from an anthropogenically impacted Mediterranean lagoon. Notably, the authors emphasized the role of ammonia in causing false-positive responses and highlighted the utility of bioassays in detecting the combined effects of complex mixtures of pollutants, including ammonia. Similarly, another study [65] found that total ammonia concentrations in elutriates was a major factor contributing to the observed toxicity in sea urchin embryo development tests. These studies support our conclusion that ammonium plays a key role in driving toxicity in marine sediment exposures and confirm the sensitivity of sea urchin embryo tests to this compound, even when the overall chemical contamination is considered low to moderate. Thus, our findings are consistent with observations from other Mediterranean coastal systems and further reinforce the ecological relevance of ammonium as a toxicant in sediment toxicity assessments.
These findings raise important questions for sediment quality assessment and management. At what point does naturally derived ammonium shift from a confounding factor to an actionable contaminant? The answer has direct implications for regulatory frameworks and remediation decisions, especially in systems with high organic loading.
In summary, the toxicity observed in Orbetello Lagoon sediments cannot be attributed to a single contaminant; instead, it is conditioned by the complex interplay of metal concentrations, organic matter contents, and sulfur levels. In our study, the toxicity measured with the embryotoxicity assay with P. lividus was associated mainly with ammonium and, with a lower contribution, with mercury. Accurate environmental risk assessments must account for these interactions to avoid underestimating ecological risks.
5. Conclusions
Our study demonstrates that ammonium plays a critical role in sediment toxicity in the Orbetello Lagoon, often more so than mercury. While mercury remains a concern, ammonium’s influence—whether through direct toxicity or altered metal bioavailability—cannot be ignored. These findings suggest that sediment management strategies must incorporate ammonium monitoring and consider seasonal and geochemical variability. Future research should focus on ammonium–metal interactions under different salinity and organic content regimes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nitrogen6030062/s1, Figure S1: Flowchart of the experimental design used to investigate the contribution of mercury and ammonium to sediment toxicity in the Orbetello Lagoon.
Author Contributions
Conceptualization, D.S., A.G., M.E.P. and S.M.; validation, A.S., P.A., G.T. and D.S.; formal analysis, D.S., A.G. and S.M.; investigation, G.T., P.A., V.T., A.S., S.F. and D.S.; resources, V.T. and S.F.; writing—original draft preparation, D.S.; writing—review and editing, A.G.; visualization, A.G. and M.E.P.; supervision, D.S. and S.M.; project administration, S.M. and M.E.P. All authors discussed the results and contributed to the final manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Toscana Region within the agreement between ISPRA and the Toscana Region for the actualization and completion of the surveys for the determination of reference values in the lagoon area, as provided for in the Program Agreement of 29 May 2018 and subsequent Supplementary Act of 4 October 2021 and its technical annex.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We sincerely thank Ing. Fabiano Pilato (ISPRA) for his valuable assistance with the granulometric analysis of sediment samples.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A
Appendix A.1

Table A1.
Concentrations of Hydrocarbons (mg/Kg dry weight), ∑ PAH (µg/Kg dry weight), ∑ PCBs (µg/Kg dry weight) and Sulfides (mg/Kg) measured in sediment from the Orbetello Lagoon.
Table A1.
Concentrations of Hydrocarbons (mg/Kg dry weight), ∑ PAH (µg/Kg dry weight), ∑ PCBs (µg/Kg dry weight) and Sulfides (mg/Kg) measured in sediment from the Orbetello Lagoon.
| DOS 01 | DOS 02 | DOS 03 | DOS 04 | DOS 05 | DOS 07 | DOS 08 | DOS 09 | DOS 10 | DOS 11 | DOS 12 | DOS 13 | DOS 14 | DOS 15 | DOS 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrocarbons (C > 12) | 20 | 32 | 23 | 39 | 18 | 33 | 24 | 53 | 27 | 34 | 57 | 41 | 74 | 65 | 48 |
| ∑ PAH | <LOQ | 18 | 12 | 30 | <10 | 22 | 18 | 27 | 41 | 25 | 27 | 18 | 15 | <LOQ | 17 |
| ∑ PCBs | 1.1 ± 0.4 | 1.2 ± 0.5 | 0.86 ± 0.34 | 1.4 ± 0.57 | 0.34 ± 0.14 | 1.8 ± 0.7 | 2.0 ± 0.8 | 3.9 ± 1.6 | 3.2 ± 1.3 | 3.9 ± 1.6 | 3.4 ± 1.4 | 3.7 ± 1.5 | 6.5 ± 2.6 | 1.7 ± 0.7 | 3.5 ± 1.4 |
| Sulfides | 113.3 | 64.3 | 174.8 | 92.0 | 31.9 | 157.4 | 65.3 | 29.1 | 83.3 | 92.8 | 22.9 | 15.8 | 26.5 | 16.1 | 17.2 |
Appendix A.2

Table A2.
Granulometric composition (%) of surface sediments collected from the Orbetello Lagoon.
Table A2.
Granulometric composition (%) of surface sediments collected from the Orbetello Lagoon.
| Sample | Silt (<63 µm) | Sand (>63 µm; <2 mm) | Gravel (>2 mm) |
|---|---|---|---|
| DOS 01 | 64.19 | 35.81 | 0.00 |
| DOS 02 | 73.85 | 26.15 | 0.00 |
| DOS 03 | 82.86 | 17.14 | 0.00 |
| DOS 04 | 67.21 | 32.79 | 0.00 |
| DOS 05 | 47.77 | 52.23 | 0.00 |
| DOS 07 | 71.64 | 28.36 | 0.00 |
| DOS 08 | 55.01 | 44.99 | 0.00 |
| DOS 09 | 60.21 | 39.79 | 0.00 |
| DOS 10 | 47.68 | 52.32 | 0.00 |
| DOS 11 | 76.17 | 23.83 | 0.00 |
| DOS 12 | 37.21 | 62.79 | 0.00 |
| DOS 13 | 73.23 | 26.77 | 0.00 |
| DOS 14 | 91.17 | 8.83 | 0.00 |
| DOS 15 | 100.00 | 0 | 0.00 |
| DOS 16 | 95.68 | 4.32 | 0.00 |
Appendix A.3

Table A3.
pH, salinity (PSU), and temperature (°C) values measured in the elutriates prior to conducting the larval development bioassay with P. lividus.
Table A3.
pH, salinity (PSU), and temperature (°C) values measured in the elutriates prior to conducting the larval development bioassay with P. lividus.
| Sample | pH | Salinity (PSU) | T (°C) |
|---|---|---|---|
| DOS 01 | 8.20 | 35.4 | 17.8 |
| DOS 02 | 8.27 | 35.6 | 18.1 |
| DOS 03 | 8.19 | 34.8 | 17.9 |
| DOS 04 | 8.26 | 35.2 | 18.0 |
| DOS 05 | 8.18 | 35.4 | 17.9 |
| DOS 07 | 8.18 | 35.2 | 17.7 |
| DOS 08 | 8.20 | 35.2 | 17.8 |
| DOS 09 | 8.21 | 35.2 | 18.1 |
| DOS 10 | 8.15 | 35.4 | 17.6 |
| DOS 11 | 8.19 | 35.6 | 18.0 |
| DOS 12 | 8.16 | 35.4 | 17.9 |
| DOS 13 | 8.09 | 34.9 | 17.8 |
| DOS 14 | 8.17 | 34.9 | 17.8 |
| DOS 15 | 8.13 | 35.1 | 17.9 |
| DOS 16 | 8.17 | 35.0 | 17.9 |
References
- Ausili, A.; Bergamin, L.; Romano, E. Environmental status of Italian coastal marine areas affected by a long history of contamination. Front. Environ. Sci. 2020, 8, 34. [Google Scholar] [CrossRef]
- ISPRA. Progetto Preliminare di Bonifica Dell’area Lagunare Antistante lo Stabilimento ex-SITOCO Inclusa All’interno Della Perimetrazione del sito di Bonifica di Interesse Nazionale di Orbetello—Area Ex Sitoco; Istituto Superiore per la Protezione e la Ricerca Ambientale: Rome, Italy, 2008; p. 107. [Google Scholar]
- ISPRA. Interventi per il Risanamento Delle Aree Lagunari di Orbetello–Laguna di Levante. Premesse di Progetto; Istituto Superiore per la Protezione e la Ricerca Ambientale: Rome, Italy, 2009; p. 105. [Google Scholar]
- Sartori, D.; Macchia, S.; Gaion, A. Did you consider ammonium? A possible confounding factor in evaluating the toxicity of marine sediments. Mar. Pollut. Bull. 2024, 199, 116021. [Google Scholar] [CrossRef]
- Bellas, J.; Granmo, K.; Beiras, R. Embryotoxicity of the antifouling biocide zinc pyrithione to sea urchin (Paracentrotus lividus) and mussel (Mytilus edulis). Mar. Pollut. Bull. 2005, 50, 1382–1385. [Google Scholar] [CrossRef]
- Morroni, L.; Gaion, A.; Broccoli, A.; Ferrari, S.; Pellegrini, D.; Sartori, D. Influence of salinity on copper toxicity in Paracentrotus lividus and Arbacia lixula embryos. Water 2023, 15, 65. [Google Scholar] [CrossRef]
- Li, L.; Zhang, B.; Shi, J.; He, J.; Zhang, W.; Yan, W.; Li, H. Concurrent vanadate and ammonium abatement in a membrane biofilm reactor. Chem. Eng. J. 2022, 442, 136285. [Google Scholar] [CrossRef]
- Samperio-Ramos, G.; Hernández-Sánchez, O.; Camacho-Ibar, V.F.; Pajares, S.; Gutiérrez, A.; Sandoval-Gil, J.M.; Reyes, M.; De Gyves, S.; Balint, S.; Oczkowski, A.; et al. Ammonium loss microbiologically mediated by Fe(III) and Mn(IV) reduction along a coastal lagoon system. Chemosphere 2023, 349, 140933. [Google Scholar] [CrossRef]
- Cloutier-Mantha, L.; Harrison, P.J. Effects of sublethal concentrations of mercuric chloride on ammonium-limited Skeletonema costatum. Mar. Biol. 1980, 56, 219–231. [Google Scholar] [CrossRef]
- Pepi, M.; Leonzio, C.; Focardi, S.; Renzi, M. Production of methyl mercury by sulphate-reducing bacteria in sediments from the Orbetello lagoon in presence of high macroalgal loads. Ecol. Quest. 2020, 31, 21–40. [Google Scholar] [CrossRef]
- Protano, G.; Bianchi, S.; De Santis, M.; Di Lella, L.A.; Nannoni, F.; Salleolini, M. New geochemical data for defining origin and distribution of mercury in groundwater of a coastal area in southern Tuscany (Italy). Environ. Sci. Pollut. Res. 2023, 30, 50920–50937. [Google Scholar] [CrossRef] [PubMed]
- Mancini, L.; Miniero, R.; Beccaloni, E.; Di Domenico, K.; Lacchetti, I.; Puccinelli, C.; Carere, M. Mercury (Hg) and methylmercury (MeHg) in sediment and biota: A case study in a lagoon in Central Italy. Mar. Pollut. Bull. 2022, 175, 113308. [Google Scholar] [CrossRef]
- US-EPA EPA 823-B-01-002; Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual. U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 2001. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/collectionmanual.pdf (accessed on 18 June 2025).
- ASTM E 1391–03; Standard Guide for Collection, Storage, Characterization, and Manipulation of Sediments for Toxicological Testing. ASTM: West Conshohocken, PA, USA, 2014. Available online: https://store.astm.org/e1391-03r14.html (accessed on 6 June 2025).
- Sartori, D.; Macchia, S.; Layglon, N.; d’Onofrio, S.; Misson, B.; Piccione, M.E.; Bertolotto, R.M.; Scuderi, A.; Pilato, F.; Giuliani, S.; et al. Elutriate preparation affects embryo development test with Paracentrotus lividus: An in-depth study on the differences between two protocols and three different sediment/water mixing times. Ecotoxicol. Environ. Saf. 2021, 212, 112010. [Google Scholar] [CrossRef]
- Cicero, A.M.; Di Girolamo, I. Metodologie Analitiche di Riferimento del Programma di Monitoraggio per il Controllo dell’Ambiente Marino Costiero (Triennio 2001–2003); Ministero dell’Ambiente e della Tutela del Territorio ICRAM: Rome, Italy, 2001. Available online: https://www.isprambiente.gov.it/contentfiles/00010000/10087-metodologie.pdf/ (accessed on 17 June 2025).
- US-EPA Method 7473 (SW-846); Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry. Environmental Protection Agency: Washington, DC, USA, 1989. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/7473.pdf (accessed on 16 June 2025).
- US-EPA Method 3545A (SW-846); Pressurized Fluid Extraction (PFE), Revision 1. Environmental Protection Agency: Washington, DC, USA, 2007. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/3545a.pdf (accessed on 18 June 2025).
- US-EPA Method 8270E (SW-846); Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS). Environmental Protection Agency: Washington, DC, USA, 2014. Available online: https://www.epa.gov/sites/default/files/2020-10/documents/method_8270e_update_vi_06-2018_0.pdf (accessed on 18 June 2025).
- US-EPA Method 1668C; Chlorinated Biphenyl Congeners in Water, Soil, Sediment, Biosolids, and Tissue by HRGC/HRMS. Environmental Protection Agency: Washington, DC, USA, 2010. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/method_1668c_2010.pdf (accessed on 18 June 2025).
- Luckzak, C.; Janquin, M.-A.; Kupka, A. Simple standard procedure for the routine determination of organic matter in marine sediment. Hydrobiologia 1997, 345, 87–94. [Google Scholar] [CrossRef]
- Pagano, G.; Cipollaro, M.; Corsale, G.; Esposito, A.; Ragucci, E.; Giordano, G. The sea urchin bioassay for the assessment of damage from environmental contaminants. In Community Toxicity Testing; Cairns, J., Jr., Ed.; American Society for Testing and Materials: Philadelphia, PA, USA, 1986. [Google Scholar] [CrossRef]
- Gaion, A.; Scuderi, A.; Pellegrini, D.; Sartori, D. Arsenic exposure affects embryo development of sea urchin, Paracentrotus lividus (Lamarck, 1816). Bull. Environ. Contam. Toxicol. 2013, 91, 565–570. [Google Scholar] [CrossRef]
- US-EPA Method 7010 (SW-846); Graphite Furnace Atomic Absorption Spectrophotometry. Environmental Protection Agency: Washington, DC, USA, 1998. Available online: https://www.epa.gov/sites/default/files/2015-07/documents/epa-7010.pdf (accessed on 18 June 2025).
- Sigudla, J.; Maritz, J.E. Exploratory factor analysis of constructs used for investigating research uptake for public healthcare practice and policy in a resource-limited setting, South Africa. BMC Health Serv. Res. 2023, 23, 1423. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Visentin, D.C.; Thapa, D.K.; Hunt, G.E.; Watson, R.; Cleary, M. Exploratory factor analysis and principal component analysis in clinical studies: Which one should you use? J. Adv. Nurs. 2020, 76, 1886–1889. [Google Scholar] [CrossRef] [PubMed]
- Weide, A.C.; Beauducel, A. Varimax Rotation Based on Gradient Projection Is a Feasible Alternative to SPSS. Front. Psychol. 2019, 10, 645. [Google Scholar] [CrossRef]
- Charles, C.; Veber, P.; Delignette-Muller, M.L. MOSAIC: A web-interface for statistical analyses in ecotoxicology. Environ. Sci. Pollut. Res. Int. 2018, 25, 11295–11302. [Google Scholar] [CrossRef] [PubMed]
- Morse: Modelling Reproduction and Survival Data in Ecotoxicology. Available online: https://cran.r-project.org/web/packages/morse/index.html (accessed on 16 June 2025).
- Italian Ministerial Decree 173/2016. Regulation Containing Technical Procedures and Criteria for the Authorization of Seabed Excavation Material and Sea (in Italian), Italian Official Journal No. 208/2016. Available online: http://www.gazzettaufficiale.it/eli/id/2016/09/06/16G00184/sg (accessed on 20 July 2025).
- Arizzi Novelli, A.; Picone, M.; Losso, C.; Volpi Ghirardini, A. Ammonia as confounding factor in toxicity tests with the sea urchin Paracentrotus lividus (Lmk). Toxicol. Environ. Chem. 2003, 85, 183–191. [Google Scholar] [CrossRef]
- Sartori, D.; Pellegrini, D.; Gaion, A. Analysis of variability in embryological response of two sea urchin species to spatial and temporal features-can these factors influence responses in standardized ecotoxicological assays? EOEB 2016, 5, S1-002. [Google Scholar] [CrossRef]
- Lenzi, M.; Renzi, M.; Nesti, U.; Gennaro, P.; Persia, E.; Porrello, S. Vegetation cyclic shift in eutrophic lagoon: Assessment of dystrophic risk indices based on standing crop evaluations. Estuar. Coast. Shelf Sci. 2013, 132, 99–107. [Google Scholar] [CrossRef]
- Lenzi, M.; Cianchi, F. Summer dystrophic criticalities of non-tidal lagoons: The case study of a Mediterranean lagoon. Diversity 2022, 14, 771. [Google Scholar] [CrossRef]
- McGlathery, K. Macroalgal blooms contribute to the decline in seagrasses in nutrient-enriched coastal waters. J. Phycol. 2001, 37, 453–456. [Google Scholar] [CrossRef]
- Lenzi, M.; Palmieri, R.; Porrello, S. Restoration of the eutrophic Orbetello Lagoon (Tyrrhenian Sea, Italy): Water quality management. Mar. Pollut. Bull. 2003, 46, 1540–1548. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment. Canadian Council of Ministers of the Environment. Canadian sediment quality guidelines for the protection of aquatic life: Polycyclic aromatic hydrocarbons (PAHs). In Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999; Available online: https://ccme.ca/en/res/polycyclic-aromatic-hydrocarbons-pahs-canadian-sediment-quality-guidelines-for-the-protection-of-aquatic-life-en.pdf (accessed on 18 June 2025).
- Canadian Council of Ministers of the Environment. Canadian Council of Ministers of the Environment. Canadian sediment quality guidelines for the protection of aquatic life: Polychlorinated biphenyls (PCBs). Updated. In Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2001; Available online: https://ccme.ca/en/res/polychlorinated-biphenyls-pcbs-canadian-sediment-quality-guidelines-for-the-protection-of-aquatic-life-en.pdf (accessed on 18 June 2025).
- Chalhoub, M.; Amalric, L.; Touzé, S.; Gallé, P.; Reiller, P.E.; Doucet, N.; Clozel, B.; Bataillard, P. PCB partitioning during sediment remobilization—A 1D column experiment. J. Soils Sediments 2013, 13, 1284–1300. [Google Scholar] [CrossRef]
- Gdaniec-Pietryka, M.; Mechlińska, A.; Wolska, L.; Gałuszka, A.; Namieśnik, J. Remobilization of polychlorinated biphenyls from sediment and its consequences for their transport in river waters. Environ. Monit. Assess. 2013, 185, 4449–4459. [Google Scholar] [CrossRef]
- Kumari, K.M.; Lakhani, A. PAHs in Gas and Particulate Phases: Measurement and Control. In Environmental Chemistry for a Sustainable World; Springer: Singapore, 2018; pp. 43–75. [Google Scholar] [CrossRef]
- Leoni, L.; Sartori, F.; Nicolai, I. Metalli pesanti nei sedimenti attuali della piattaforma costiera Toscana. Atti Soc. Toscana Sci. Nat. Mem. A. 1993, 102, 23–60. [Google Scholar]
- Bengtsson, H.; Alvenäs, G.; Nilsson, S.I.; Hultman, B.; Öborn, I. Cadmium, copper and zinc leaching and surface run-off losses at the Öjebyn farm in Northern Sweden—Temporal and spatial variation. Agric. Ecosyst. Environ. 2006, 113, 120–138. [Google Scholar] [CrossRef]
- Fernandez, N.; Beiras, R. Combined toxicity of dissolved mercury with copper, lead and cadmium on embryogenesis and early larval growth of the Paracentrotus lividus sea-urchin. Ecotoxicology 2001, 10, 263–271. [Google Scholar] [CrossRef]
- Cesar, A.; Marín-Guirao, L.; Vita, R.; Marín, A. Sensitivity of Mediterranean amphipods and sea urchins to reference toxicants. Cienc. Mar. 2002, 28, 407–417. [Google Scholar] [CrossRef]
- Novelli, A.A.; Losso, C.; Ghetti, P.F.; Volpi Ghirardini, A. Toxicity of heavy metals using sperm cell and embryo toxicity bioassays with Paracentrotus lividus (Echinodermata: Echinoidea): Comparisons with exposure concentrations in the Lagoon of Venice, Italy. Environ. Toxicol. Chem. 2003, 22, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Pasquetti, F.; Vaselli, O.; Zanchetta, G.; Nisi, B.; Lezzerini, M.; Bini, M.; Mele, D. Sedimentological, Mineralogical and Geochemical Features of Late Quaternary Sediment Profiles from the Southern Tuscany Hg Mercury District (Italy): Evidence for the Presence of Pre-Industrial Mercury and Arsenic Concentrations. Water 2020, 12, 1998. [Google Scholar] [CrossRef]
- Cossa, D.; Coquery, M. The Mediterranean mercury anomaly, a geochemical or a biological issue. In The Mediterranean Sea. Handbook of Environmental Chemistry; Saliot, A., Ed.; Springer: Berlin, Germany, 2005; Volume 5K, pp. 177–208. [Google Scholar] [CrossRef]
- Rimondi, V.; Gray, J.E.; Costagliola, P.; Vaselli, O.; Lattanzi, P. Concentration, distribution, and translocation of mercury and methylmercury in mine-waste, sediment, soil, water, and fish collected near the Abbadia San Salvatore Mercury Mine, Monte Amiata District, Italy. Sci. Total Environ. 2012, 414, 318–327. [Google Scholar] [CrossRef]
- Lattanzi, P.; Rimondi, V.; Chiarantini, L.; Colica, A.; Benvenuti, M.; Costagliola, P.; Ruggieri, G. Mercury dispersion through streams draining the Mt. Amiata District, southern Tuscany, Italy. Procedia Earth Planet. Sci. 2017, 17, 468–471. [Google Scholar] [CrossRef]
- Pontoni, L.; La Vecchia, C.; Boguta, P.; Sirakov, M.; D’Aniello, E.; Fabbricino, M.; Locascio, A. Natural organic matter controls metal speciation and toxicity for marine organisms: A review. Environ. Chem. Lett. 2022, 20, 797–812. [Google Scholar] [CrossRef]
- Besser, J.M.; Brumbaugh, W.G.; May, T.W.; Ingersoll, C.G. Effects of organic amendments on the toxicity and bioavailabil-ity of cadmium and copper in spiked formulated sediments. Environ. Toxicol. Chem. 2003, 22, 805–815. [Google Scholar] [CrossRef]
- De Schamphelaere, K.A.C.; Unamuno, V.I.R.; Tack, F.M.G.; Vanderdeelen, J.; Janssen, C.R. Reverse osmosis sampling does not affect the protective effect of dissolved organic matter on copper and zinc toxicity to freshwater organisms. Chemosphere 2005, 58, 653–658. [Google Scholar] [CrossRef]
- Smith, K.S.; Ranville, J.F.; Lesher, E.K.; Diedrich, D.J.; McKnight, D.M.; Sofield, R.M. Fractionation of fulvic acid by iron and aluminum oxides-Influence on copper toxicity to Ceriodaphnia dubia. Environ. Sci. Technol. 2014, 48, 11934–11943. [Google Scholar] [CrossRef] [PubMed]
- Al-Reasi, H.A.; Scott, D.S.; Wood, C.M. Evaluating the ameliorative effect of natural dissolved organic matter (DOM) quality on copper toxicity to Daphnia magna: Improving the BLM. Ecotoxicology 2012, 21, 524–537. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z. Interactions of Zn(II) ions with humic acids isolated from various types of soils: Effect of pH, Zn concentrations and humic acids chemical properties. PLoS ONE 2016, 11, e0153626. [Google Scholar] [CrossRef]
- Bai, H.; Jiang, Z.; He, M.; Ye, B.; Wei, S. Relating Cd2⁺ binding by humic acids to molecular weight: A modeling and spectroscopic study. J. Environ. Sci. 2018, 70, 154–165. [Google Scholar] [CrossRef]
- Damikouka, I.; Katsiri, A. Chemical Speciation and Heavy Metal Mobility in Contaminated Marine Sediments. In Contaminated Sediments: Sustainable Management and Remediation; Galvez, R., Dyer, M., Eds.; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar] [CrossRef]
- Vicinie, A.; Palermo, M.; Matko, L. A review of the applicability of various elutriate tests and refinements of these methodologies. In Proceedings of the Western Dredging Association (WEDA XXXI) Technical Conference & Texas A&M University (TAMU 41) Dredging Seminar, Houston, TX, USA, 6–9 June 2011; Available online: https://www.westerndredging.org/phocadownload/ConferencePresentations/2011_Nashville/Session1A-DredgingResearch/4%20-%20Vicinie%20Palermo%20Matko%20-%20Review%20of%20Applicability%20of%20Various%20Elutriate%20Tests%20Refinements%20of%20Methods.pdf (accessed on 18 June 2025).
- Ravichandran, M. Interactions between mercury and dissolved organic matter—A review. Chemosphere 2004, 55, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, L.; Carvan III, M.J.; Guo, L. Mitigative effects of natural and model dissolved organic matter with different functionalities on the toxicity of methylmercury in embryonic zebrafish. Environ. Pollut. 2019, 252, 616–626. [Google Scholar] [CrossRef]
- Aukema, K.G. Bioavailability of hydrophobic organic contaminants in sediments with different particle-size distributions. Arch. Environ. Contam. Toxicol. 2011, 61, 74–82. [Google Scholar] [CrossRef]
- Eggleton, J.; Thomas, K.V. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ. Int. 2004, 30, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, C.; Valdehita, A.; Vethaak, A.D.; Navas, J.M.; León, V.M. Toxicity characterization of surface sediments from a Mediterranean coastal lagoon. Chemosphere 2020, 253, 126710. [Google Scholar] [CrossRef] [PubMed]
- Picone, M.; Bergamin, M.; Losso, C.; Delaney, E.; Novelli, A.A.; Ghirardini, A.V. Assessment of sediment toxicity in the Lagoon of Venice (Italy) using a multi-species set of bioassays. Ecotoxicol. Environ. Saf. 2016, 123, 32–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).