Soybean (Glycine Max L.) Grain Yield Response to Inoculation with Novel Bradyrhizobia Strains Across Different Soil Fertility Conditions in Zimbabwe
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Rhizobia Strain Details
2.2. Trial Establishment and Setup
2.3. Soil Sampling and Soil Chemical Analysis
2.4. Plant Material Sampling and Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Site Soil Characteristics
3.2. Nodulation Counts and Nodule Mass of Soybeans During Flowering at the Researcher-Managed Sites
3.3. Biomass Production of Soybean During Flowering at the Researcher-Managed Sites
3.4. Soybean Grain Yields at All Sites
3.5. Nitrogen Uptake and Estimated Nitrogen Derived from Fixation at Site 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SPRL | Soil Productivity Research Laboratory |
| CSRI | Chemistry and Soil Research Institute |
| HRI | Horticultural Research Institute |
| KPC | Kushinga Phikelela Agricultural College |
| SNF | symbiotic nitrogen fixation |
| OC | organic carbon |
References
- Giller, K.E. The successful intensification of smallholder farming in Zimbabwe. LEISA Mag. 2008, 24, 30–31. [Google Scholar]
- FAOSTAT. Crops and Livestock Products; Food and Agriculture Organization of the United Nations: Rome, Italy, 2025; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 5 June 2025).
- Basera, J.; Mushoriwa, H. Soyabean: A strategic crop. In Field Crops; Seed Co Zimbabwe: Harare, Zimbabwe, 2025; Available online: https://www.seedcogroup.com/zw/fieldcrops/soyabean-a-strategic-crop/#:~:text=The%20country%20requires%20about%20220,basket%20for%20the%20general%20populace (accessed on 5 June 2025).
- Zambon, L.M.; Umburanas, R.C.; Schwerz, F.; Sousa, J.B.; Barbosa, E.S.T.; Inoue, L.P.; Dourado-Neto, D.; Reichardt, K. Nitrogen balance and gap of a high yield tropical soybean crop under irrigation. Front. Plant Sci. 2023, 14, 1233772. [Google Scholar] [CrossRef] [PubMed]
- Desta, M.; Akuma, A.; Minay, M.; Yusuf, Z.; Baye, K. Effects of indigenous and commercial rhizobia on growth and nodulation of soybean (Glycine max L.) under greenhouse condition. Open Biotechnol. J. 2023, 17. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, M.; Goyal, R.; Gill, B.S. Physical characteristics and nutritional composition of some new soybean (Glycine max (L.) Merrill) genotypes. J. Food Sci. Technol. 2014, 51, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Nzeyimana, F.; Onwonga, R.N.; Ayuke, F.O.; Chemining’wa, G.N.; Nabahungu, N.L.; Bigirimana, J.; Noella Josiane, U.K. Determination of abundance and symbiotic effectiveness of native rhizobia nodulating soybean and other legumes in Rwanda. Plant Environ. Interact. 2024, 5, e10138. [Google Scholar] [CrossRef] [PubMed]
- Syromiatnykov, Y.; Yakovlieva, A.; Voinash, S.; Orekhovskaya, A.; Vanzha, V.; Akhtyamova, L.; Vashchilin, V. Efficiency of symbiosis between Bradyrhizobium bacteria and soybean plants under various biopreparations. BIO Web Conf. 2025, 181, 01028. [Google Scholar] [CrossRef]
- Hungriaa, M.; Vargas, M.A.T. Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Res. 2000, 65, 151–164. [Google Scholar] [CrossRef]
- Tumbure, A.; Dube, S.; Tauro, T.P. Insights of microbial inoculants in complementing organic soil fertility management in African smallholder farming systems. In Towards Sustainable Food Production in Africa; Springer: Singapore, 2023; pp. 59–83. [Google Scholar]
- Jarecki, W.; Borza, I.M.; Rosan, C.A.; Vicas, S.I.; Domuța, C.G. Soybean response to seed inoculation with Bradyrhizobium japonicum and/or nitrogen fertilization. Agriculture 2024, 14, 1025. [Google Scholar] [CrossRef]
- Kanonge-Mafaune, G.; Chiduwa, M.S.; Chikwari, E.; Pisa, C. Evaluating the effect of increased rates of rhizobial inoculation on grain legume productivity. Symbiosis 2018, 75, 217–227. [Google Scholar] [CrossRef]
- Chekanai, V.; Chikowo, R.; Vanlauwe, B. Response of common bean (Phaseolus vulgaris L.) to nitrogen, phosphorus and rhizobia inoculation across variable soils in Zimbabwe. Agric. Ecosyst. Environ. 2018, 266, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Chibeba, A.M.; Kyei-Boahen, S.; Guimaraes, M.F.; Nogueira, M.A.; Hungria, M. Feasibility of transference of inoculation-related technologies: A case study of evaluation of soybean rhizobial strains under the agro-climatic conditions of Brazil and Mozambique. Agric. Ecosyst. Environ. 2018, 261, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Zengeni, R.; Mpepereki, S.; Giller, K.E. Manure and soil properties affect survival and persistence of soyabean nodulating rhizobia in smallholder soils of Zimbabwe. Appl. Soil. Ecol. 2006, 32, 232–242. [Google Scholar] [CrossRef]
- Chiduwa, M. Improving the Legume-rhizobium Symbiosis in Zimbabwean Agriculture: A Study of Rhizobia Diversity & Symbiotic Potential Focussed on Soybean Root Nodule Bacteria. Ph.D. Dissertation, Murdoch University, Murdoch, Australia, 2021. [Google Scholar]
- Szczerba, A.; Plazek, A.; Kopec, P.; Wojcik-Jagla, M.; Dubert, F. Effect of different Bradyrhizobium japonicum inoculants on physiological and agronomic traits of soybean (Glycine max (L.) Merr.) associated with different expression of nodulation genes. BMC Plant Biol. 2024, 24, 1201. [Google Scholar]
- Ndhlovu, K.; Bopape, F.L.; Diale, M.O.; Mpai, T.; Morey, L.; Mtsweni, N.P.; Gerrano, A.S.; Vuuren, A.v.; Babalola, O.O.; Hassen, A.I. Characterization of nodulation-compatible strains of native soil rhizobia from the rhizosphere of Soya bean (Glycine max L.) Fields in South Africa. Nitrogen 2024, 5, 1107–1123. [Google Scholar] [CrossRef]
- Omari, R.A.; Yuan, K.; Anh, K.T.; Reckling, M.; Halwani, M.; Egamberdieva, D.; Ohkama-Ohtsu, N.; Bellingrath-Kimura, S.D. Enhanced soybean productivity by inoculation with indigenous Bradyrhizobium strains in agroecological conditions of northeast Germany. Front. Plant Sci. 2021, 12, 707080. [Google Scholar] [CrossRef] [PubMed]
- Musiyiwa, K.; Mpepereki, S.; Giller, K.E. Physiological diversity of rhizobia nodulating promiscuous soyabean in Zimbabwean soils. Symbiosis 2005, 40, 97–107. [Google Scholar]
- FAO. Fertilizer Use by Crop in Zimbabwe; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2006. [Google Scholar]
- Anderson, J.; Ingram, J. Tropical soil biology and fertility: A handbook of methods. Soil Sci. 1994, 157, 265. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Okalebo, R.J.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual, 2nd ed.; TSBF-CIAT and SACRED Africa, Kenya: Nairobi, Kenya, 2002. [Google Scholar]
- Tumbure, A.; Wuta, M.; Mapanda, F. Preliminary evaluation of the effectiveness of Rhizobium leguminosarum bv. viceae strains in nodulating hairy vetch (Vicia villosa) in the sandy soils of Zimbabwe. S. Afr. J. Plant Soil 2013, 30, 233–239. [Google Scholar] [CrossRef]
- Wang, W.; Shi, F.; Du, J.; Li, L.; Bai, T.; Xing, X. Soil factors that contribute to the abundance and structure of the diazotrophic community and soybean growth, yield, and quality under biochar amendment. Chem. Biol. Technol. Agric. 2023, 10, 54. [Google Scholar] [CrossRef]
- Shen, D.; Bisseling, T. Soybean breeders can count on nodules. Trends Plant Sci. 2025, 30, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alla, M.H.; Al-Amri, S.M.; El-Enany, A.-W.E. Enhancing Rhizobium–legume symbiosis and reducing nitrogen fertilizer use are potential options for mitigating climate change. Agriculture 2023, 13, 2092. [Google Scholar] [CrossRef]
- Zubrod, M. Counting the nodules that count, relationships between seed nitrogen and root nodules. Nat. Sci. Educ. 2022, 51, e20088. [Google Scholar] [CrossRef]
- Chikwanha, H. High production costs render soyabean uncompetitive. In The Herald; Zimpapers Ltd.: Harare, Zimbabwe, 2024; Available online: https://www.heraldonline.co.zw/high-production-costs-render-soyabean-uncompetitive/#:~:text=Zimbabwe%27s%20average%20soya%20bean%20yield,2%2C4%20tonnes%20per%20ha. (accessed on 30 May 2025).
- Omondi, J.O.; Chiduwa, M.S.; Kyei-Boahen, S.; Masikati, P.; Nyagumbo, I. Yield gap decomposition: Quantifying factors limiting soybean yield in southern Africa. NPJ Sustain. Agric. 2024, 2, 32. [Google Scholar] [CrossRef]
- Manzeke-Kangara, M.G.; Ligowe, I.S.; Tibu, A.; Gondwe, T.N.; Greathead, H.M.R.; Galdos, M.V. Soil organic carbon and related properties under conservation agriculture and contrasting conventional fields in northern Malawi. Front. Soil Sci. 2025, 4, 1481275. [Google Scholar] [CrossRef]
- Nyawasha, R.W.; Falconnier, G.N.; Todoroff, P.; Wadoux, A.M.J.C.; Chikowo, R.; Coquereau, A.; Leroux, L.; Jahel, C.; Corbeels, M.; Cardinael, R. Drivers of soil organic carbon stocks at village scale in a sub-humid region of Zimbabwe. Catena 2025, 252, 108843. [Google Scholar] [CrossRef]
- Swanepoel, P.A.; Botha, P.R.; Truter, W.F.; Surridge-Talbot, A.K. The effect of soil carbon on symbiotic nitrogen fixation and symbiotic Rhizobium populations in soil with Trifolium repensas host plant. Afr. J. Range Forage Sci. 2011, 28, 121–127. [Google Scholar] [CrossRef]
- Nezomba, H.; Mtambanengwe, F.; Tittonell, P.; Mapfumo, P. Practical assessment of soil degradation on smallholder farmers’ fields in Zimbabwe: Integrating local knowledge and scientific diagnostic indicators. Catena 2017, 156, 216–227. [Google Scholar] [CrossRef]
- Chipomho, J.; Rugare, J.T.; Mabasa, S.; Zingore, S.; Mashingaidze, A.B.; Chikowo, R. Short-term impacts of soil nutrient management on maize (Zea mays L.) productivity and weed dynamics along a toposequence in eastern Zimbabwe. Heliyon 2020, 6, e05223. [Google Scholar] [CrossRef] [PubMed]
- Mutuma, S.P.; Okello, J.J.; Karanja, N.K.; Woomer, P.L. Smallholder farmers’ use and profitability of legume inoculants in western Kenya. Afr. Crop Sci. J. 2014, 22, 205–213. [Google Scholar]
- GRDC. Soybean nutrition and fertiliser. In GRDC Grownotes; Grains Research and Development Corporation: Barton, ACT, Australia, 2016. [Google Scholar]
- Kebonye, N.M.; John, K.; Delgado-Baquerizo, M.; Zhou, Y.; Agyeman, P.C.; Seletlo, Z.; Heung, B.; Scholten, T. Major overlap in plant and soil organic carbon hotspots across Africa. Sci. Total Environ. 2024, 951, 175476. [Google Scholar] [CrossRef] [PubMed]
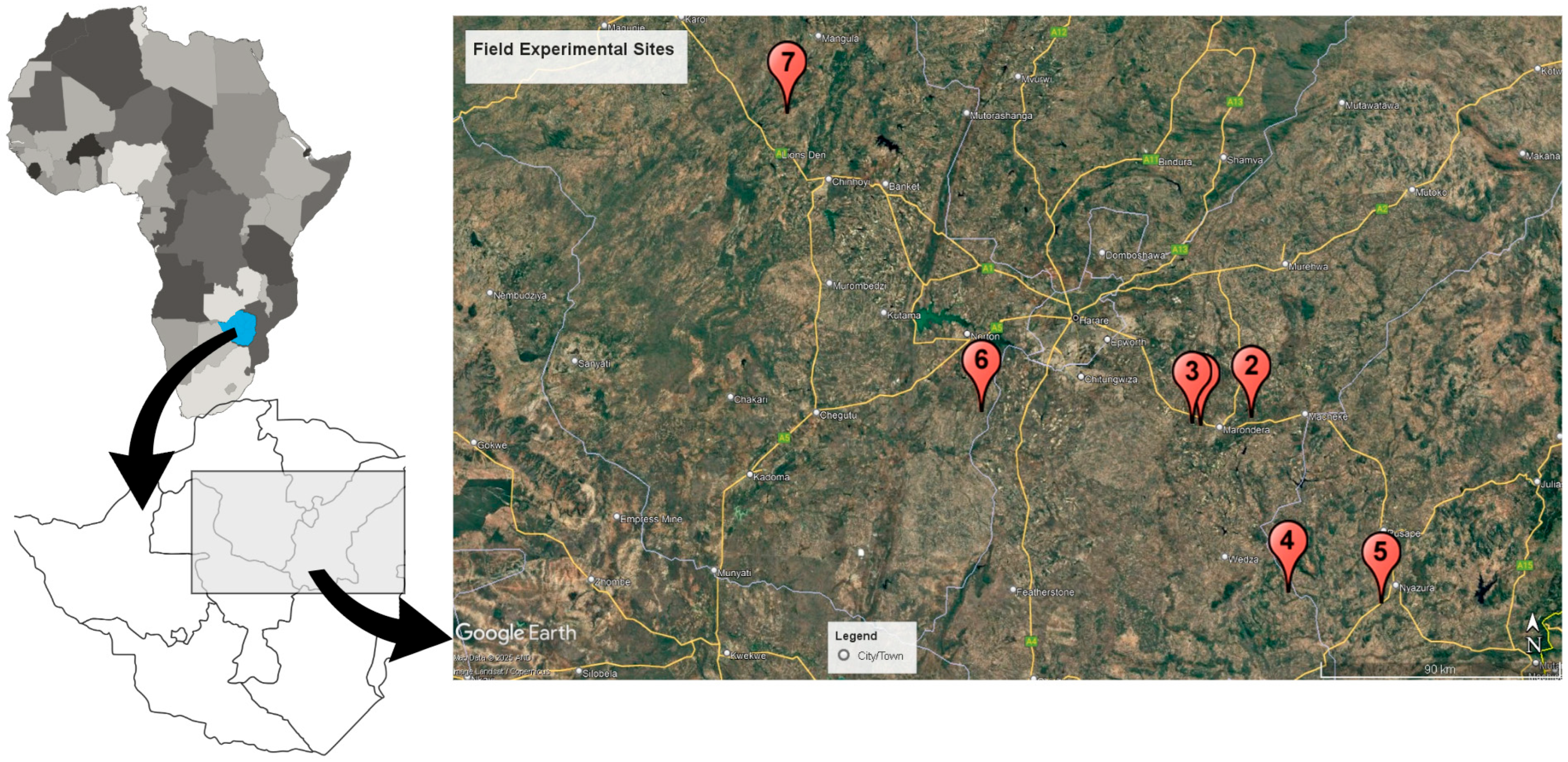
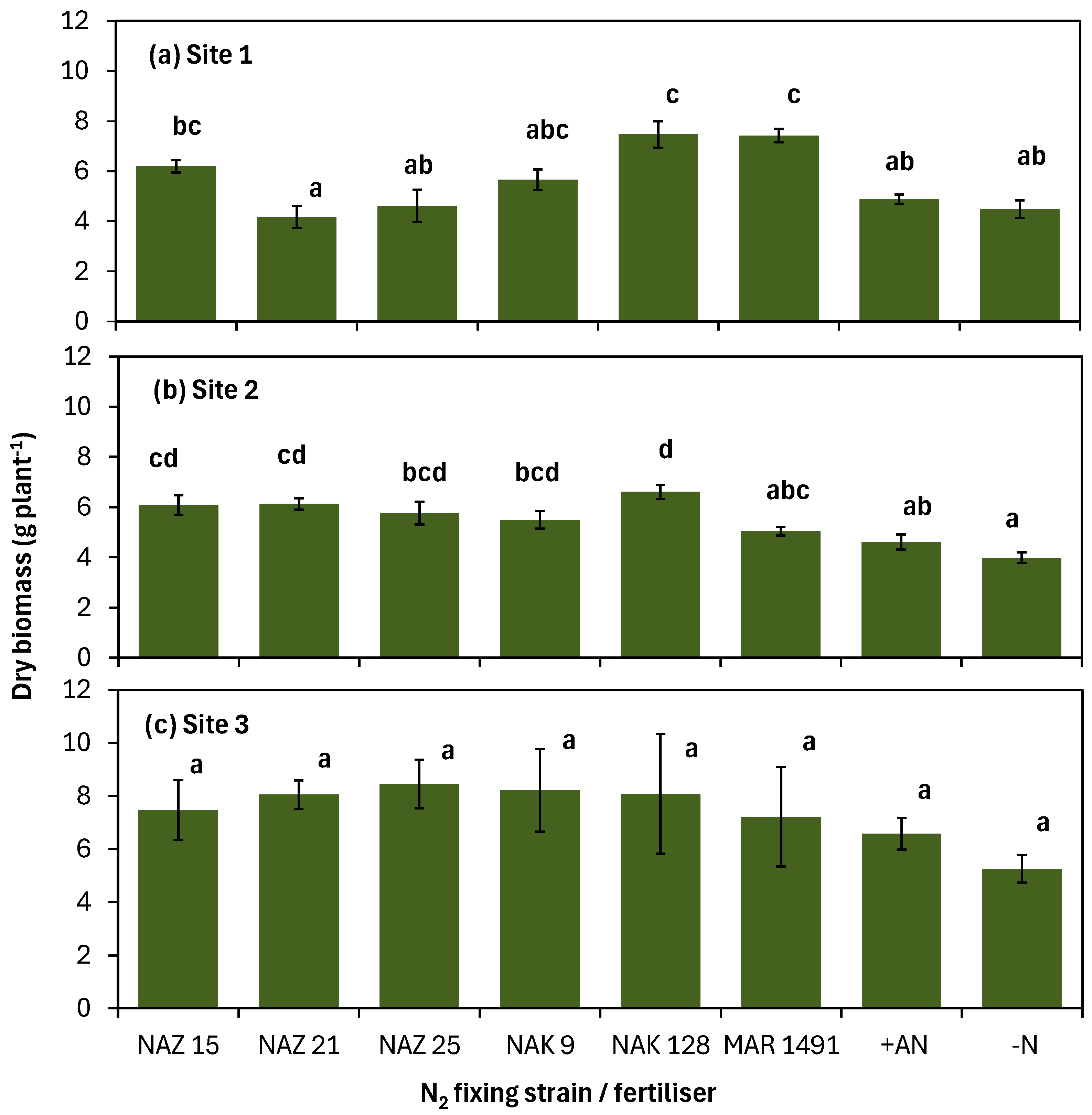
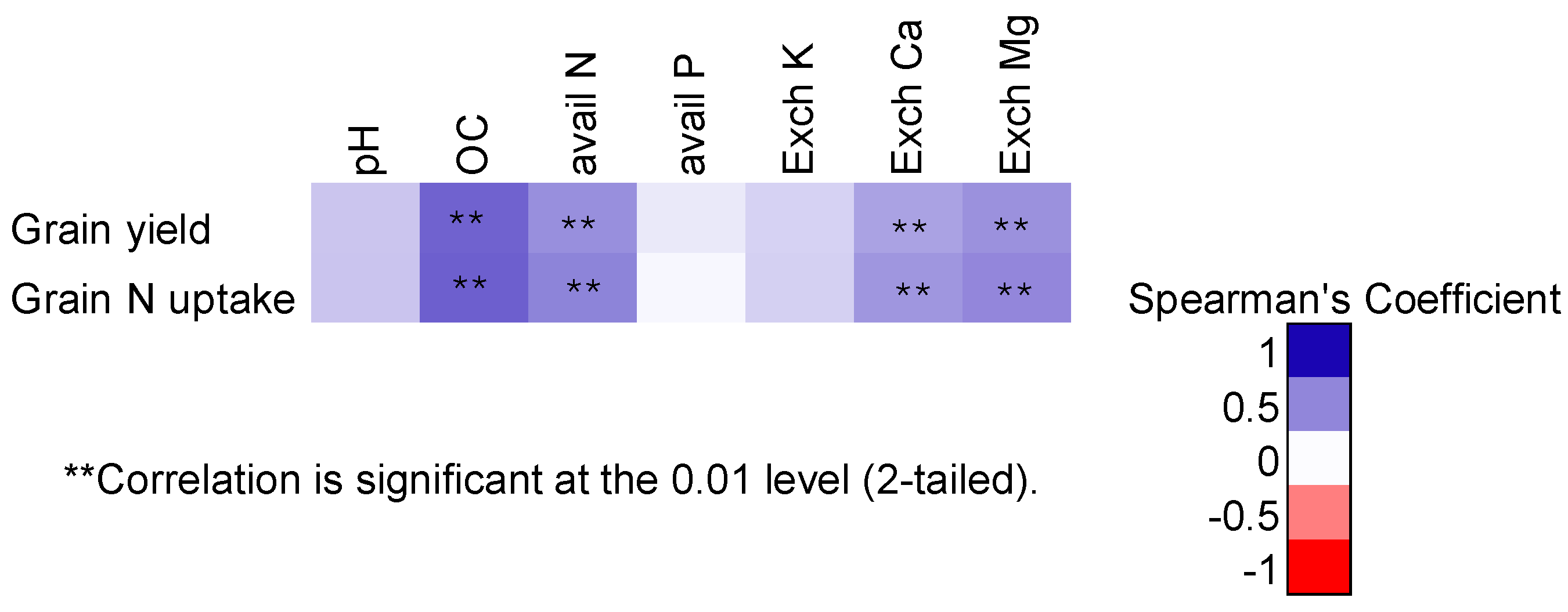
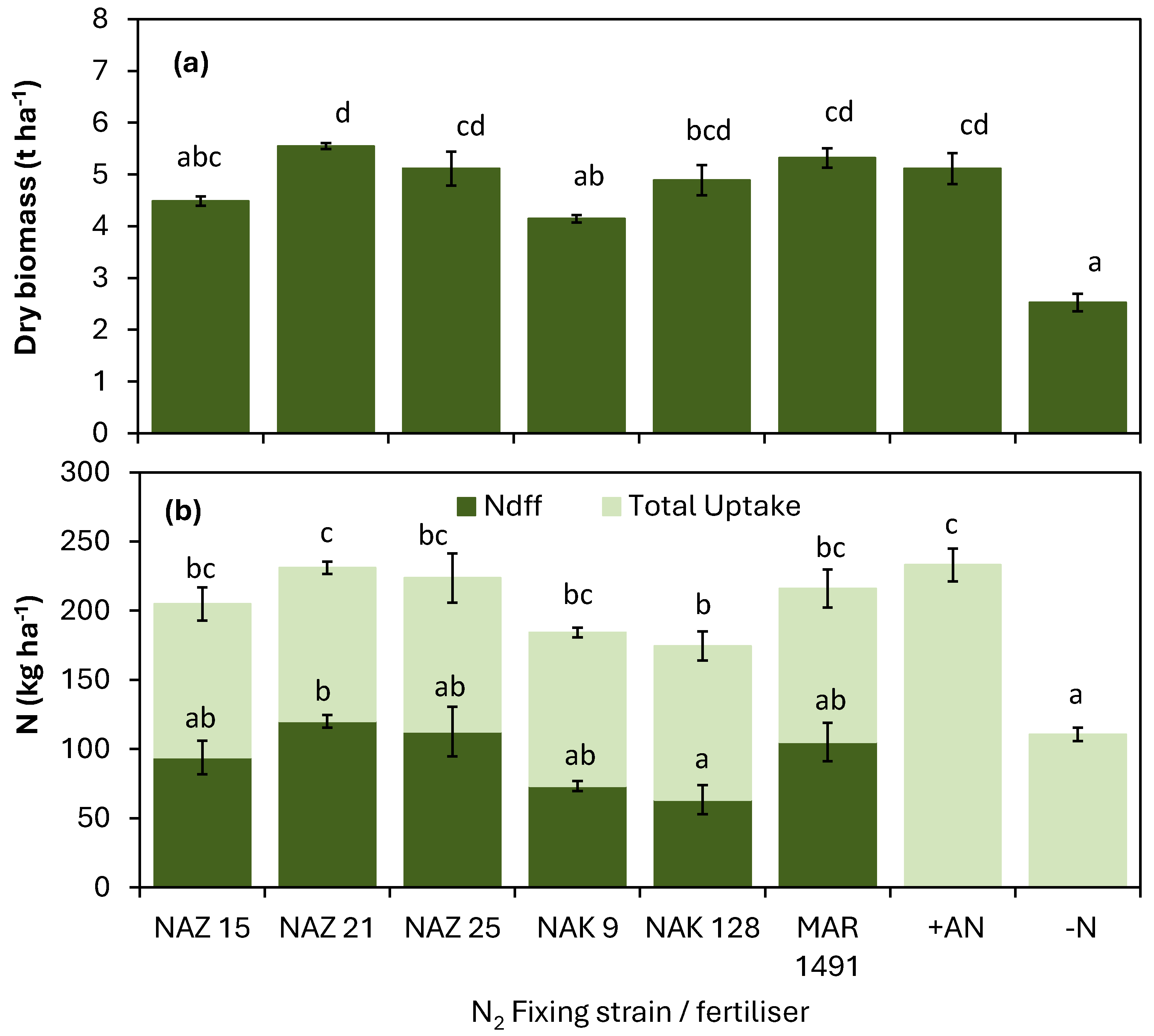
| Site | Details/Names | pH 1 | Soil OC% | Mineral N (ppm) | Avail. P (ppm) | Exchangeable Bases (me%) | ||
|---|---|---|---|---|---|---|---|---|
| K | Ca | Mg | ||||||
| 1 | SPRL | 4.5 | 0.35 | 22 | 5 | 0.09 | 0.64 | 0.38 |
| 2 | Kushinga Phikelela College | 5.3 | 1.08 | 11 | 37 | 0.42 | 2.4 | 1.51 |
| 3 | Horticulture Research Institute | 4.9 | 1.28 | 23 | 19.6 | 1.02 | 3.63 | 0.20 |
| 4 | Wedza | 5.0 | 0.23 | 5 | 4 | 0.05 | 0.73 | 0.35 |
| 5 | Rusape/Makoni | 5.1 | 1.35 | 44 | 2 | 0.25 | 9.41 | 4.1 |
| 6 | Mhondoro | 4.7 | 1.06 | 29 | 2 | 0.56 | 5.35 | 2.83 |
| 7 | Chinhoyi | 5.6 | 1.20 | 28 | 9 | 0.74 | 6.43 | 4.3 |
| Site 1 | Site 2 | Site 3 | ||||
|---|---|---|---|---|---|---|
| N-Fixing Strain/Fertilizer | Nodules Plant−1 | Fresh Nodule Mass Plant−1 (g) | Nodules Plant−1 | Fresh Nodule Mass Plant−1 (g) | Nodules Plant−1 | Fresh Nodule Mass Plant−1 (g) |
| NAZ15 | 36 d ± 1 | 0.98 c ± 0.07 | 25 d ± 1 | 0.38 bc ± 0.09 | 25 b ± 2 | 0.79 cd ± 0.11 |
| NAZ21 | 24 bcd ± 2 | 0.57 ab ± 0.06 | 13 cd ± 1 | 0.38 bc ± 0.05 | 10 a ± 2 | 0.42 abc ± 0.05 |
| NAZ25 | 7 ab ± 1 | 0.59 ab ± 0.03 | 11 abc ± 1 | 0.40 bc ± 0.04 | 17 ab ± 4 | 0.82 cd ± 0.16 |
| NAK9 | 17 abc ± 2 | 0.60 ab ± 0.05 | 11 abc ± 1 | 0.22 b ± 0.02 | 10 a ± 2 | 0.43 abc ± 0.04 |
| NAK128 | 27 cd ± 4 | 0.84 bc ± 0.07 | 12 bcd ± 1 | 0.43 c ± 0.04 | 22 ab ± 2 | 1.00 d ± 0.10 |
| MAR1491 | 24 bcd ± 1 | 0.88 bc ± 0.04 | 16 cd ± 1 | 0.23 bc ± 0.04 | 21 ab ± 2 | 0.73 bcd ± 0.10 |
| +AN | 1 a ± 0 | 0.00 a ± 0 | 0.4 ab ± 0 | 0.04 a ± 0.02 | 12 a ± 4 | 0.37 a ± 0.05 |
| -N | 2 a ± 1 | 0.15 a ± 0.06 | 0.1 a ± 0 | 0.01 a ± 0.01 | 10 a ± 2 | 0.40 ab ± 0.08 |
| Significance | ** | ** | ** | *** | ** | *** |
| N-Fixing Strain/Fertilizer | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 |
|---|---|---|---|---|---|---|---|
| Grain Yield (t ha−1) | |||||||
| NAZ15 | 1.84 b ± 0.14 | 1.47 b ± 0.08 | 3.31 b ± 0.21 | 0.81 b ± 0.09 | 2.15 ab ± 0.16 | 1.06 ± 0.04 | 1.12 a ± 0.12 |
| NAZ21 | 1.31 ab ± 0.17 | 2.10 c ± 0.07 | 3.28 b ± 0.10 | 0.86 b ± 0.13 | 2.56 b ± 0.22 | 1.13 ± 0.14 | 1.63 ab ± 0.11 |
| NAZ25 | 1.70 b ± 0.12 | 1.63 b ± 0.06 | 3.27 b ± 0.25 | 1.08 b ± 0.16 | 2.41 b ± 0.30 | 1.28 ± 0.21 | 1.73 ab ± 0.24 |
| NAK9 | 1.08 a ± 0.12 | 1.45 ab ± 0.09 | 2.43 a ± 0.13 | 0.70 b ± 0.08 | 2.88 b ± 0.17 | 1.30 ± 0.23 | 1.60 ab ± 0.19 |
| NAK128 | 1.69 b ± 0.16 | 1.59 b ± 0.12 | 2.90 ab ± 0.07 | 0.77 b ± 0.06 | 2.34 b ± 0.18 | 0.75 ± 0.08 | 0.83 a ± 0.16 |
| MAR1491 | 1.63 ab ± 0.14 | 1.70 bc ± 0.13 | 3.26 b ± 0.17 | 0.63 ab ± 0.10 | 1.99 ab ± 0.28 | 1.59 ± 0.30 | 2.35 b ± 0.36 |
| +AN | 1.83 b ± 0.08 | 1.75 bc ± 0.08 | 3.47 b ± 0.18 | 0.30 ab ± 0.03 | 2.29 b ± 0.25 | 1.07 ± 0.16 | 1.61 ab ± 0.15 |
| -N | 1.39 ab ± 0.06 | 1.02 a ± 0.08 | 1.61 a ± 0.07 | 0.16 a ± 0.06 | 1.23 a ± 0.19 | 0.77 ± 0.15 | 1.49 ab ± 0.20 |
| Significance | *** | *** | * | *** | *** | ns | * |
| Grain N Uptake (kg N / ha−1) | |||||||
| NAZ15 | 107.7 b ± 7.6 | 86.4 b ± 4.2 | 106.20 b ± 7.95 | 52.4 c ± 5.4 | 145.2 ab ± 13.6 | 62.1 ± 7.6 | 70.9 ab ± 11.6 |
| NAZ21 | 77.1 ab ± 9.8 | 103.0 b ± 8.6 | 106.87 b ± 6.60 | 58.7 c ± 10.3 | 156.2 b ± 14.3 | 60.9 ± 6.3 | 93.0 ab ± 5.9 |
| NAZ25 | 99.1 ab ± 7.2 | 95.5 b ± 7.2 | 104.58 b ± 9.72 | 64.4 c ± 7.3 | 148.3 ab ± 16.4 | 73.6 ± 12.8 | 103.8 ab ± 14.5 |
| NAK9 | 61.6 a ± 6.4 | 68.2 ab ± 9.0 | 77.33 b ± 3.72 | 42.7 bc ± 5.1 | 182.4 b ± 12.6 | 71.6 ± 17.3 | 100.3 ab ± 13.8 |
| NAK128 | 99.4 ab ± 9.3 | 92.9 b ± 12.9 | 66.35 ab ± 10.33 | 41.8 bc ± 4.8 | 141.4 ab ± 15.4 | 37.6 ± 4.8 | 52.9 a ± 11.5 |
| MAR1491 | 95.0 ab ± 8.0 | 94.4 b ± 6.3 | 97.25 b ± 8.30 | 41.3 bc ± 7.8 | 121.0 ab ± 21.9 | 96.1 ± 21.2 | 126.5 b ± 20.9 |
| +AN | 113.8 b ± 2.7 | 86.8 b ± 6.5 | 106.91 b ± 7.58 | 17.3 ab ± 1.9 | 150.1 b ± 15.4 | 51.2 ± 5.7 | 100.2 ab ± 13.8 |
| -N | 67.8 a ± 12.9 | 43.4 a ± 3.4 | 50.80 a ± 1.45 | 10.0 a ± 3.6 | 76.9 a ± 11.5 | 42.7 ± 10.7 | 91.4 ab ± 15.4 |
| Significance | * | *** | *** | *** | *** | ns | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumbure, A.; Kanonge, G.; Mukungurutse, C.S.; Mushangwe, C.; Tauro, T.P.; Chiduwa, M.S. Soybean (Glycine Max L.) Grain Yield Response to Inoculation with Novel Bradyrhizobia Strains Across Different Soil Fertility Conditions in Zimbabwe. Nitrogen 2025, 6, 59. https://doi.org/10.3390/nitrogen6030059
Tumbure A, Kanonge G, Mukungurutse CS, Mushangwe C, Tauro TP, Chiduwa MS. Soybean (Glycine Max L.) Grain Yield Response to Inoculation with Novel Bradyrhizobia Strains Across Different Soil Fertility Conditions in Zimbabwe. Nitrogen. 2025; 6(3):59. https://doi.org/10.3390/nitrogen6030059
Chicago/Turabian StyleTumbure, Akinson, Grace Kanonge, Collis S. Mukungurutse, Cathrine Mushangwe, Tonny P. Tauro, and Mazvita S. Chiduwa. 2025. "Soybean (Glycine Max L.) Grain Yield Response to Inoculation with Novel Bradyrhizobia Strains Across Different Soil Fertility Conditions in Zimbabwe" Nitrogen 6, no. 3: 59. https://doi.org/10.3390/nitrogen6030059
APA StyleTumbure, A., Kanonge, G., Mukungurutse, C. S., Mushangwe, C., Tauro, T. P., & Chiduwa, M. S. (2025). Soybean (Glycine Max L.) Grain Yield Response to Inoculation with Novel Bradyrhizobia Strains Across Different Soil Fertility Conditions in Zimbabwe. Nitrogen, 6(3), 59. https://doi.org/10.3390/nitrogen6030059





